Research summary
Even the simplest cognitive task requires communication between multiple sensory, motor, and association areas. Communication between these multiple areas is mediated by long-range projections acting on local neurons and is modulated by brain states. Our main research interest is to understand the adaptive changes in the properties of neural circuits at multiple spatial levels during the sensitive-periods of neurodevelopment and maladaptive changes associated with disease states.
We have been using the rodent whisker-barrel somatosensory and olfacotory system to understand the organizing principles of cortical circuits and decipher the way they integrate into different circuits in the thalamocortical and sensorimotor cortex. We are interested in understanding how local and long-ranges cortical circuits are constructed, as well as how they adapt to sensory experiences and disease states. Our focus has been on GABA releasing inhibitory cells, because GABAergic synapses provide an inhibitory tone to local circuits, thereby determining the final output of local circuits. Distinct inhibitory neurons interact with excitatory neurons in space and time; they differentially contribute to the regulation of network oscillations and circuit dynamics. Although the phenomena of functional differences in different interneurons have been measured across many brain regions, the mechanisms that underlie different activation patterns have been poorly understood.
Abnormal inhibitory circuit wiring in cortical neurons are the key features of social and cognitive dysfunction such as epilepsy, autism and schizophrenia. Diverse genetic mutations cause different developmental brain disorders (DBD), yet many syndromes share similar intellectual and cognitive impairments. A common feature of these impairments involves disrupted vigilance and brain states. Thus, major efforts of my lab for the following decades will be to investigate how neural circuits, especially long-range circuits, undergo maladaptive changes in disease states across different states of consciousness. Due to the complexity of neurodevelopmental disorders, we have prosed an muti-level approch research statergy. Our approches are designed to provide better understanding of the etiology and mechanisms of DBD. The multi-level approch is summarized in the graph below.
Research philosophy
Because the complexity of the brain network in terms of both its connectivity and plasticity, it is important to gain understanding at multiple level of neuronal organizations. We use so called multilevel investigatoion approches, which are briefly sumarized in this figure.
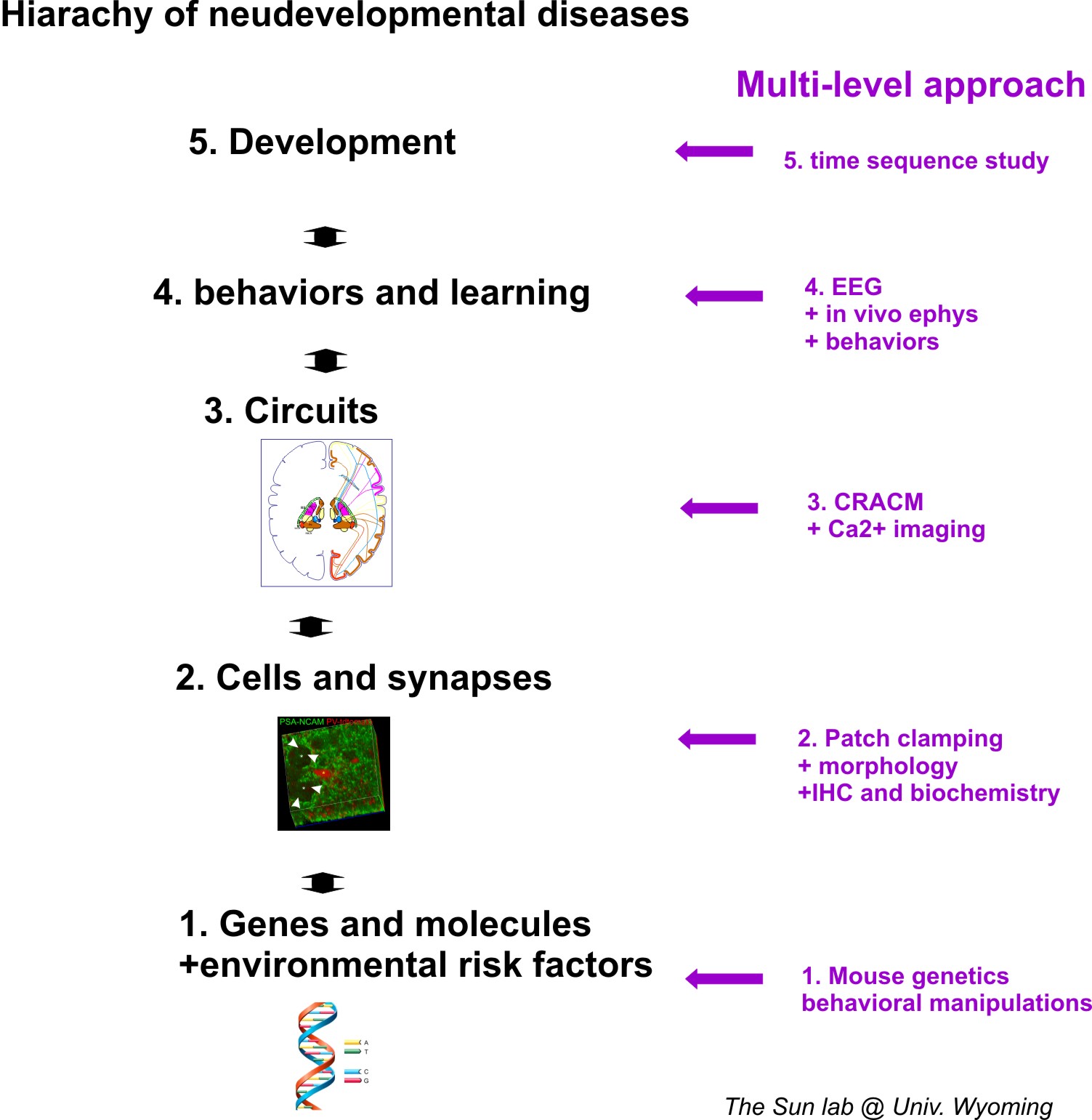
Research interests highlights
-Ion channels, Synapses and Circuits.
-Brain waves and oscillations.
-Neurodevelopmental disorders (Auism, schizophrenia, cortical malformation and epsilepsy).
Research tools
Because the complexity of the brain network in terms of both its connectivity and plasticity, it is important to gain understanding at multiple level of neuronal organizations. We use so called multilevel investigatoion approches, which are briefly sumarized in this table.
Table: multi-level approches used in Dr Sun's lab.
Multilevel approaches used in Sun lab |
1. Single cell labeling and morphometric analysis |
2. Brain slice patch clamp rig |
3.Channel-rhodopsin 2 assisted circuit mapping (CRACM) |
4.EEG in freely behaving animals 24/hrs |
5.In vivo/single cell recording in head fixed mouse rig (New) |
6.GCaMP/Ca2+ imaging in head fixed mouse rig (New) |
7. Rodent behavior assays |
Scientific goals |
Cellular and structural properties |
Synapse and intrinsic properties; E/I balance |
Long-range and local neural circuit mapping in normal, epileptic and developmental disorders |
Brain waves /seizure detection oscillations and coherence |
Cellular mechanisms underlying sensory processing in vivo |
Network activities underlies sensory processing in vivo |
Locomotion; sleep wake cycle; Social interactions; Cognition and learning. |
Here are some examples of each of these approches currently implemented in Dr. Sun's lab.
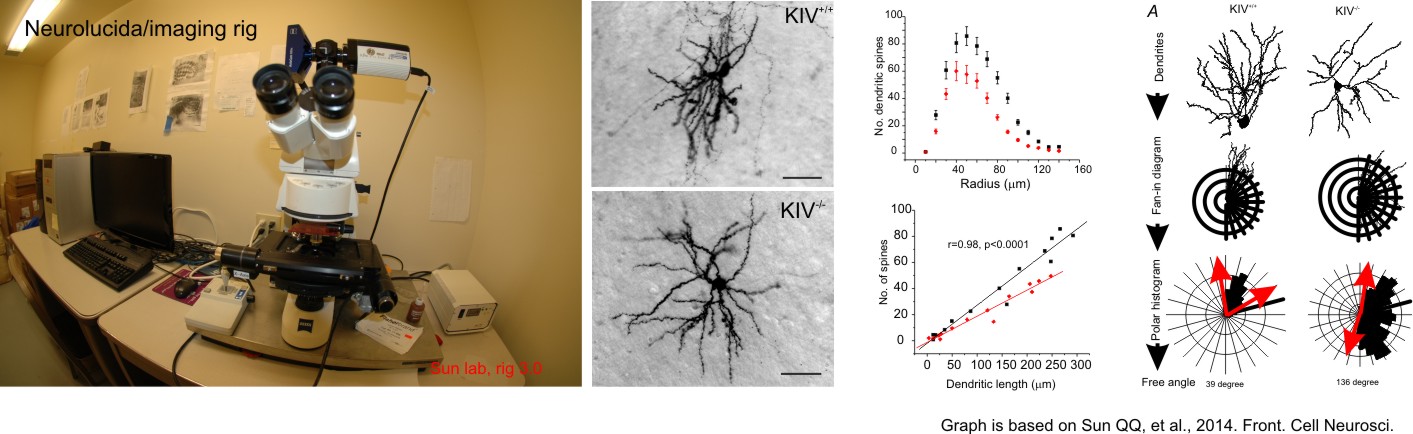
1. Single cell labeling and morphometric analysis.
a) We use Neurolucida program, which is a powerful tool for creating and analyzing realistic, meaningful, and quantifiable neuron reconstructions from microscope images. Perform detailed morphometric analysis of neurons, such as quantifying: the number of dendrites, axons, nodes, synapses, and spines the length, width, and volume of dendrites and axons the area and volume of the soma the complexity and extension of neurons. Sun lab use this program extensively and our research has led to the discovery of new-neuron types, and understand the mechanisms associated with neurodevelopmental disorders. An example is shown below.
b) We also use confocal Z-stack photos to analysize the relationship between single or group of dye or genetically labeled neurons.
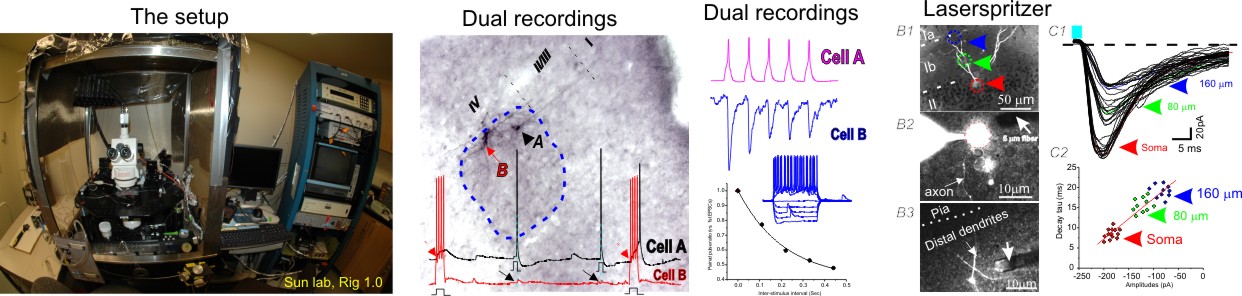
2. Brain slice patch-clamp recordings and laserspritzers for optogenetic interogations.
There are a number of configurations of whole-cell or cell-attached patch clamp recordings we often use, these includes: variance mean-analysis, seperation of excitatory vs. inhibitory synatic conductances of evoked mixed synaptic responses, intrisic and excitability analysis, paired recordings and unitary analysis, etc. These recording modes are often combined with the optogenetic tools. We developed a refined fiber optic based stimulation method (laserspritzer) which enable us to stimulate neurons with a impressive resolution of less than 20 micron (Sun QQ et al., 2014, Plos one).
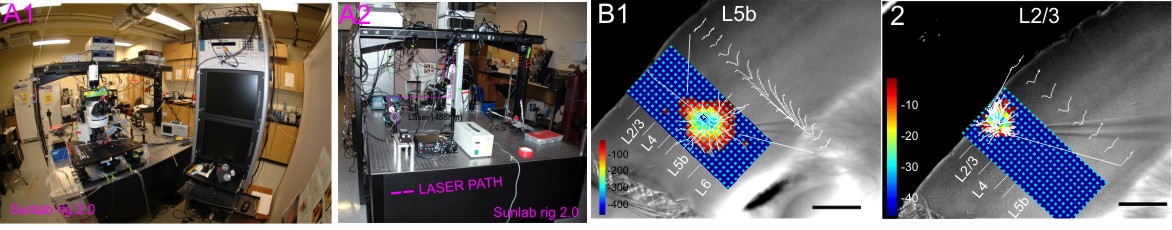
3. Channel-rhodopsin 2 assisted circuit mapping (CRACM) in brain slices.
CRACM is a powerful tool for functional mapping of neural circuits and long-range connections using ChR2 photo-stimulation developed by Dr Karl Svoboda's lab at HHMI. Photo-activation of ChR2 is performed by shuttering the beam of a blue laser (470nm) in the specimen plane via a 5x objective. The movement of the laser beam is precisely controlled with mirror galvanometers, triggered by scanning and data acquisition software Ephus (http://www.ephus.org). A user defined mapping grid which has row and column spacing of 50 μm will be applied for recordings. An example of CRACM set-up and results are shown.
4. EEG recordings and optogenetic/chemogenetic manipulation in freely behaving mouse over 24/hour period.
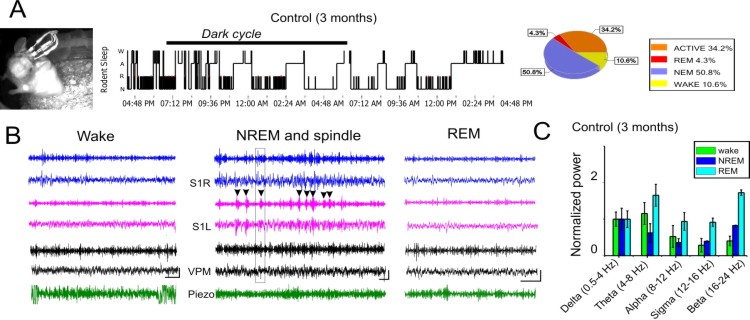
A screw free, glue-based electrode assembly system that allows for long-term recordings are used. This system was developed by Dr Liang Zhang at Toronto Western Research Institute. A reference electrode is placed into the ipsilateral olfactory bulb area. EEG recordings are performed in a 24 hour cycle with simultaneous video behavior monitoring and automated infrared (IR)-activity tracking at a frequency of twice per month to minimize disturbances. Animals are to move freely in a recording chamber supplied with water gel. EEG signals are amplified via a differential AC amplifier (Model 1700, A-M system), digitized using Power 1401, and analyzed using the Spike-2 program (Cambridge Electronic Design). Offline analysis is conducted using custom programs developed using Matlab®. Overnight EEG will yield large amounts of data, which will be used to assess the integrity of ongoing neural activity.Power spectrum. We first estimate the power in several frequency ranges (δ:1-4 Hz, θ:4-8 Hz, α:8-12 Hz and β:16-24 Hz) for each electrode. All EEG power is normalized to its baseline levels during the NREM sleep stage.
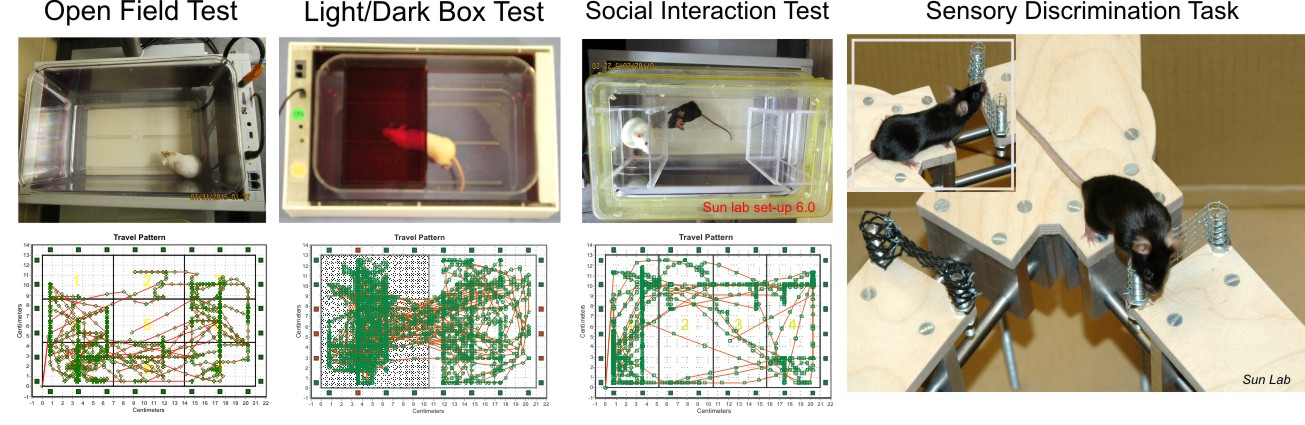
5. Automated and unautomated rodent behaviour studies.
We use an automated modular mice behavior testing platform (smartcage) for use with a standard mouse home cage. The different add-on modules (piezo sensor, dark box, sociability enclosures, and floor foot shock) enable a wide range of behavioral assays depending on the research question of interest. The automatic data collection parameters include travel plots, active counts, travel distance, rear up counts, and velocity.
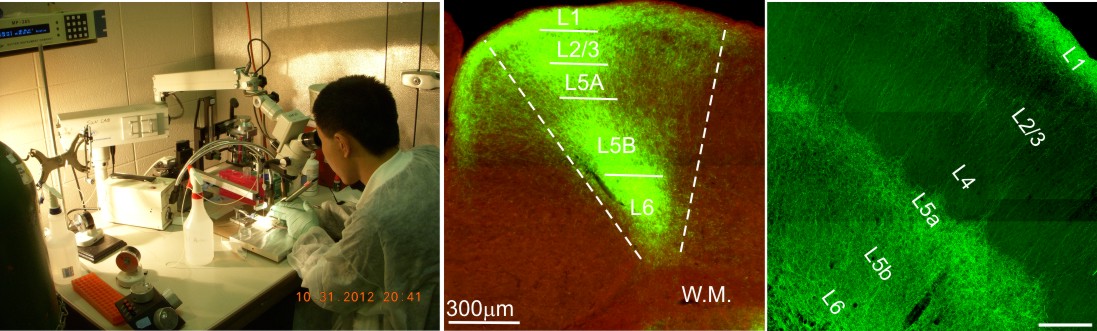
6. Transfection of brain cells with viruses in vivo.
This approch enable us to deliever recombinant DNA (such as genes for fluorescent protein marker, or genetically coded calcium indicator, or light/ligand -sensitive ion channels) directly into specific brain regions. Upon full expression of the viral vector, these brain regions (or their targeting regions) can be manipulated with light/ligand, or oberved using sensitive imaging tools.
Past research projects
1. Inhibitory circuits of the somatosensory cortex. The first part of our research has been focused on understanding the fundamental physiological and anatomical properties of cortical GABAergic inhibitory circuits in the somatosensory cortex. We and others have identified the importance of layer IV parvalbumin-expressing, fast spiking cells in mediating thalamocortical feed-forward inhibitions, and how gap-junctions between these cells are involved in thalamocortical feedforward inhibitions. We studied how these cells and another type of interneuron known as the regular spiking non-pyramidal cells (RSNP), are differentially modulated by metabotropic glutamate receptor subtypes and NMDA receptor subtypes (NR2A vs.BR2B). We contributed to collaborative research that led to the understanding of the mechanisms of interneurons in neural coding during active somatosensation, revealed using illusory touch.
- Sun QQ, Huguenard JR, Prince DA. (2006) Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. Journal of Neuroscience. 25; 26(4):1219-30.
- Sun QQ*, Zhang Z, Jiao Y, Zhang C, Szabó G, Erdelyi F. (2009a) Differential metabotropic glutamate receptor expression and modulation in two neocortical inhibitory networks. Journal of Neurophysiology. 101(5):2679-92.
- Zhang Z, Jiao YY, Sun QQ*. (2011e) Developmental maturation of excitation and inhibition balance in principal neurons across four layers of somatosensory cortex. Neuroscience.174:10-25.
- O'Connor DH, Hires SA, Guo ZV, Li N, Yu J, Sun QQ, Huber D, Svoboda K. (2013a) Neural coding during active somatosensation revealed using illusory touch. Nature Neuroscience. 16:958-65.
2. Mechanisms underlying experience-dependent plasticity of cortical inhibitory circuits during the postnatal sensitive period in normal and disease states. Using the so-called “barrel cortex” as a model system, we contributed to the understanding of the mechanisms underlying activity-dependent regulation of neocortical inhibitory circuits during postnatal development. We have identified activity-dependent synthesis of brain-deprived trophic factor (BDNF) as the key player underlying the maturation of parvalbumin positive FS but not RSNP cells. We further defined the mechanisms leading to intrinsic and synaptic plasticity of fast-spiking cells, and how the maturation of these cells maintains balance with excitation during postnatal period. Applying this knowledge, we are comparing the maturation of FS cells associated with disease states. We identified the parvalbumin cell as a key target underlying fragile-X syndrome, and neocortical malformation. In a NMDA hypo-function model of schizophrenia, we demonstrated the important role of NR2A subunits in the developmental plasticity of fast-spiking interneurons during critical periods.
- Jiao Y, Zhang C, Yanagawa Y, Sun QQ*. (2006b) Major effects of sensory experiences on the neocortical inhibitory circuits. Journal of Neuroscience. 23; 26 (34): 8691-701.
- Jiao Y, Zhang Z, Zhang C, Wang X, Sakata K, Lu B, Sun QQ*. (2011d) A key mechanism underlying sensory experience-dependent maturation of neocortical GABAergic circuits in vivo. PNAS. 108:12131-6.
- Sun QQ*. (2009b) Experience-dependent intrinsic plasticity in interneurons of barrel cortex layer IV. Journal of Neurophysiology. 102(5):2955-73.
- Zhang Z and Sun QQ* (2011a) Development of NMDA NR2 subunits and their role in critical period maturation of neocortical GABAergic interneurons. Developmental. Neurobiology. 71:221-45.
3. Inhibitory circuits of the olfactory cortex. This second part of research has been focused on understanding the fundamental physiological and anatomical properties of cortical GABAergic inhibitory circuits in the olfactory (i.e. piriform cortex) cortex. Within the piriform cortex, we systematically characterized the intrinsic and firing properties of different types of interneurons and described how they form functional circuits within local glutamatergic cells. Recently, we developed an innovative optogenetic approach (laserspritzer) to understand the physiological properties of a unique synapse: axon-axonic synapses in the olfactory cortex. Together our work has led to the construction of an inhibitory diagram within the olfactory cortex.
- Wang X and Sun QQ*. (2014a) Thorough GABAergic Innervation of the Entire Axon Initial Segment Revealed by an Optogenetic Laserspritzer. Journal of Physiology (London). 592:4257-76.
- Wang X, Sun QQ*. (2012) Characterization of axo-axonic synapses in the piriform cortex of Mus musculus. J Comp Neurol. 520(4):832-47.
- Young A, Sun QQ* (2009c) GABAergic Inhibitory Interneurons in the Posterior Piriform Cortex of the GAD67-GFP Mouse. Cerebral Cortex. 19(2):3011-29.
- Zhang CZ; Szabó G, Erdélyi F, Rose JD & Sun QQ* (2006c) A Novel Interneuronal Network in the Mouse Posterior Piriform Cortex. Journal of Comparative Neurology. 499(6):1000-15.
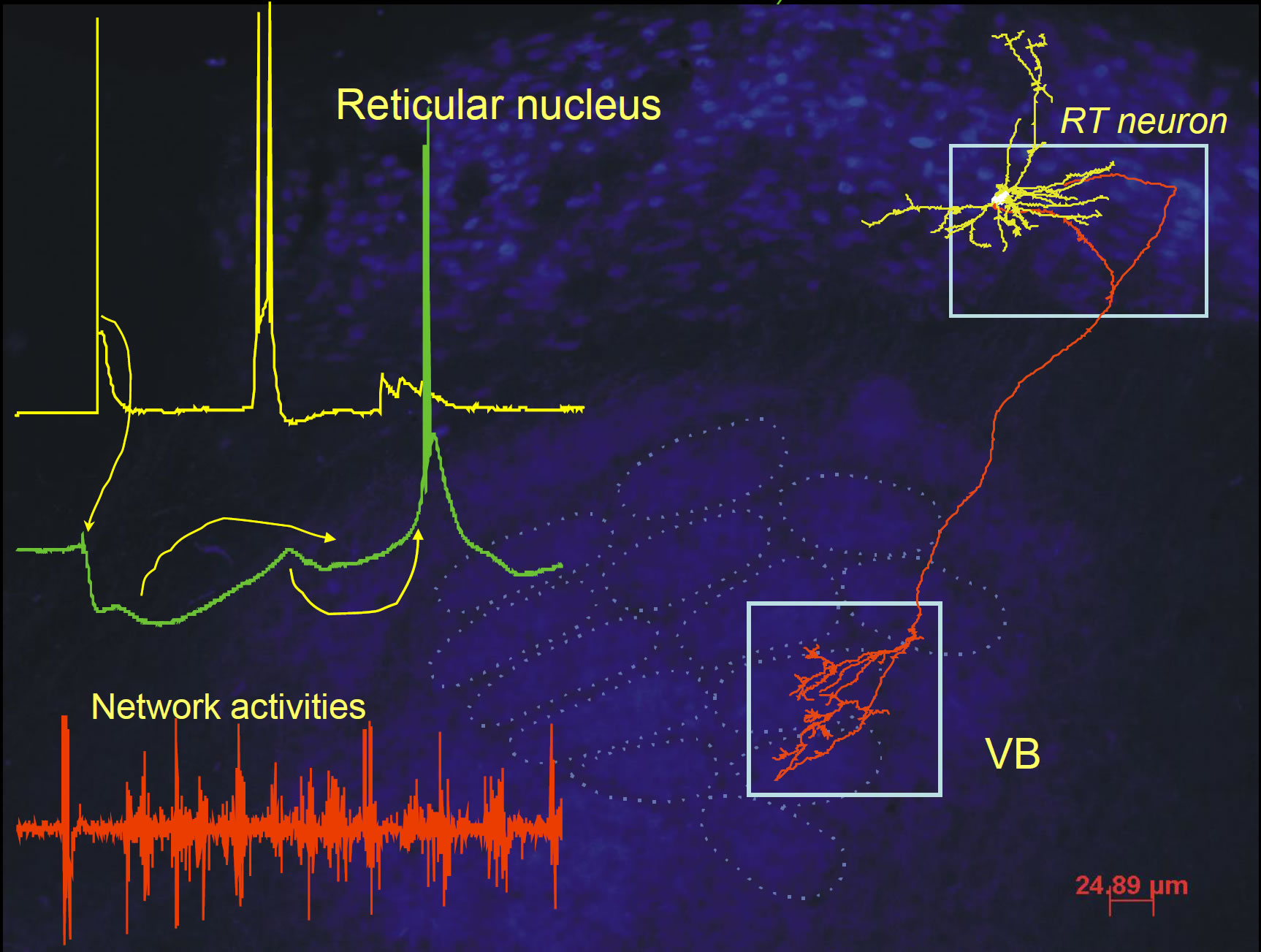
4. Thalamocortical synchrony, the role of endogenous neuropeptides as endogenous anticonvulsant. During Dr. Sun's postdoctoral training period at Stanford, his collaborative work led to the understanding of the role of several key endogenous neuropeptides, including neuropeptide Y (NPY), Vasoactive intestinal polypeptide (VIP), pituitary adenylate cyclase-activating polypeptide (PACAP), and somatostatins (SST), in modulation of thalamocortical synchrony. Thalamocortical synchrony is tightly linked to the sleep-wake cycle and absence epilepsy. Their work has led to the concept of endogenous anticonvulsant in absence epilepsy.
- Sun QQ, Baraban SC, Prince DA and Huguenard JR (2003a) Target-specific NPYergic synaptic inhibition and its network consequences within the mammalian thalamus Journal of Neuroscience 23: 9639-9649.
- Sun QQ, Prince DA and Huguenard JR (2003b) Vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide activate hyperpolarization-activated cationic current and depolarize thalamocortical neurons in vitro. Journal of Neuroscience 23: 2751-8.
- Sun QQ, Huguenard JR and Prince DA. (2002) Somatostatin inhibits thalamic network oscillations in vitro: actions on the GABAergic neurons of the reticular nucleus. Journal of Neuroscience 22: 5374-5386.
- Sun QQ, Huguenard JR and Prince DA. (2001) Neuropeptide Y receptors differentially modulate G-protein-activated inwardly rectifying K+ channels and high-voltage-activated Ca2+ channels in rat thalamic neurons. The Journal of Physiology (London), 531(1): 67-79.
5. Serotonergic modulation of voltage-gated calcium channels. During Dr Sun's doctoral training, he and his mentor (Dr. N. Dale) studied the mechanisms underlying serotonergic modulation of sensory and motor neurons in spinal neurons of Xenopus tadpoles. My contribution has been delineating the different G-protein pathways with kinetics changes of calcium channel gating. Their work has helped us identify a novel signaling pathway of serotonergic receptors.
- Sun QQ & Dale N (1999) G-Proteins are involved in 5-HT receptor-mediated modulation of N- and P/Q- but not T-type Ca2+ channels. Journal of Neuroscience 19(3): 890-9.
- Sun QQ, & Dale N (1998a) Differential inhibition of N and P/Q Ca2+ currents by 5-HT1A and 5-HT1D receptors in spinal neurons of Xenopus larvae. Journal of Physiology (London), 510 (1): 103-20.
- Sun QQ & Dale N. (1998b) Developmental changes in ion channel expression accompany maturation of locomotor pattern in frog tadpoles. Journal of Physiology (London) 507 (1): 257-264.
- Sun QQ & Dale N. (1997) Serotonergic Inhibition of T-type and High voltage-activated Ca2+ currents in the primary sensory neurons of Xenopus larvae. Journal of Neuroscience. 17(18): 6839-6849.
