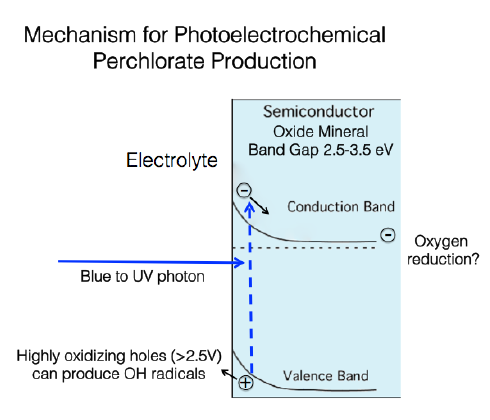
Perchlorate Formation on Mars
Analysis of Martian soil has shown perchlorate, ClO4-, is the most abundant anion in Mars’ soil. If perchlorate is forming on mars by the same mechanism as it is on Earth, we would expect 5 to 8 orders of magnitude less perchlorate on Mars. So how is it forming? The Parkinson lab is proposing a photoelectrochemical mechanism of the formation of perchlorate on Mars, whereby semiconducting metals oxides on Mars’ surface absorb the UV light, forming a highly reducing electron and a highly oxidizing hole, which then oxidizes chloride to perchlorate. We are funded by NASA to explore the UV photochemical oxidation of chlorides to perchlorate via hydroxide radicals. We are constructing an environmental chamber that mimics Martian temperatures, pressure, and gas composition characterize the mechanism of perchlorate production.