Two UW Professors Explore Mitotic Spindle Assembly in Paper Published in Science
November 14, 2013

Every cell, no matter how large or small, must regulate the size of its internal parts. Like organs, these parts, called organelles, perform basic functions to keep cells alive and healthy.
University of Wyoming researchers Jay Gatlin and John Oakey have developed a research method to address this question of how one such organelle, the mitotic spindle, scales with changes in cell size.
If organelles are too big or too small, they might not function properly, resulting in cell death, or worse, transformation into a cancerous cell. If these errors occur during embryonic development, birth defects may be the result. In other words, size does matter. But how organelles “know” how big to be is a question that biologists have been asking for generations.
Gatlin, a UW assistant professor of molecular biology, and Oakey, a UW assistant professor of chemical and petroleum engineering, are using egg extract in African clawed frogs, in combination with microfluidic technology, to take a closer look at the regulation of organelle size -- specifically mitotic spindle assembly -- within cell cytoplasm.
The two faculty members brought their expertise together in an interdisciplinary collaboration that will result in publication of a paper in this month’s issue of Science, an international scientific journal. Gatlin is lead writer of the paper, titled “Changes in Cytoplasmic Volume are Sufficient to Drive Spindle Sealing.” He scribed the paper with Oakey and UW doctoral students James Hazel of Gilbert, Ariz., Kaspars Krutkamelis of Laramie and Paul Mooney of Valparaiso, Ind.; Miroslav Tomschik, a UW senior research scientist; and Ken Gerow, professor and head of the UW Department of Statistics.
Mitotic spindles, shaped similar to the American football, are responsible for correctly segregating or separating chromosomes during cell division. Cytoplasm is the gel-like substance inside the enclosed membrane of a biological cell.
“If you make cytoplasmic droplets of different sizes, the size of the spindle assembled within them will adjust proportionally. By changing the amount of cytoplasm these spindles are assembled in, we were able to mimic or copy spindle scaling as it occurs during development,” Gatlin says. “It seems fundamental, but how this size regulation is achieved is a question that has gone unanswered for a long time.”
Gatlin was able to control the size, shape and composition of the droplets using a microfluidic device called a droplet generator, which Oakey customized for Gatlin’s research. Microfluidics is the control and manipulation of fluids that are geometrically constrained at the sub-millimeter scale.
 “We can ask really fundamental questions about how the size of a cell affects its
fundamental workings,” Gatlin says. “The amount of cytoplasm determines how big the
spindle is and also may be involved in determining the size of other intracellular
structures as well.”
“We can ask really fundamental questions about how the size of a cell affects its
fundamental workings,” Gatlin says. “The amount of cytoplasm determines how big the
spindle is and also may be involved in determining the size of other intracellular
structures as well.”
“This technology is something that is going to be adopted immediately and used across the entire Xenopus research community,” Oakey says. “They (Xenopus researchers) use frogs to study how to treat cancer and how to prevent birth defects.”
“We hope to, one day, use what we’ve learned about spindle size regulation to develop organelle-directed, anti-cancer therapies,” Gatlin says.
From the frog
Oddly enough, the research process begins with sperm taken from African clawed male frogs. The female frogs are then injected with hormones that induce them to lay eggs. The frogs, distinguished by claws on their back legs, are ideal because they have been used for research models since the 1920s and lay copious amounts of eggs, Gatlin says. The eggs provide a rich source of cellular components, including small molecules, proteins and organelles.
Each egg starts as one cell before it divides into two cells, and then four, and so on -- all the while multiplying in an embryo whose total size remains the same. This is called “reductive division, because cell size becomes progressively smaller with each cycle of division,” Gatlin says.
“The total volume (of the embryo) doesn’t change. The cells just keep cleaving in half,” he says. “The innards of the cell have to scale. As cells get smaller, the innards have to get smaller, too.”
To isolate or separate out the cytoplasm from other components of the cells, the eggs are spun in a centrifuge. This process generates a large amount of material that is used for the experiments.
Using Oakey’s customized technology, Gatlin was able to generated cell-sized droplets of cytoplasm and assemble spindles within them.
“We can control the size of an extract droplet and induce it to progress through the cell cycle just like a cell in the body,” Gatlin explains. “In other words, one can make a droplet act just like a real cell. “
In real cells, DNA emits signals that instruct the cytoplasm to assemble microtubules, which are similar in shape to long cables or tubes. The microtubules act as scaffolding and are the major structural element of the mitotic spindle. Molecular motors move along microtubules, slide them along each other and, ultimately, assemble them into a spindle-like shape, Gatlin says.
“If a cell assembles a spindle of the wrong shape or size, its chromosomes might not be segregated properly during division, resulting in aneuploidy (an abnormal number of chromosomes within a cell) and, perhaps, cancer,” Gatlin says. “Interestingly, in many types of cancer, the normal relationship between cell size and organelle size has gone awry.”
Microfluidics provides key
Gatlin says he has pondered how to control spindle size in the past. But he says he
didn’t have his “eureka moment” until he observed Oakey’s work with microfluidics
during a UW Molecular and Cellular  Life Science program seminar. Specifically, Oakey presented a movie of water droplet
formation in an oil phase using a T-junction microfluidic device.
Life Science program seminar. Specifically, Oakey presented a movie of water droplet
formation in an oil phase using a T-junction microfluidic device.
“Without John’s technology, we couldn’t do what we’re doing,” Gatlin says. “His technology is the enabling part of the work presented in the paper.”
Oakey explains that he customized the droplet generators so that the fluids used -- chemicals and oils -- do not affect what occurs inside the droplet. He added his emulsions were unique, in that droplets could be created all at the same size.
“Once we found we could encapsulate the nuclei in drops, incubate them and control their shape, Jay worked on the ability to image them,” Oakey says. “His imaging capabilities are exquisite.”
The technology allowed for reductions in cytoplasmic volume that were sufficient to artificially repeat spindle scaling observed in the frog embryos during development.
“It turns out that by changing the volume of these droplets, we could affect the size of the spindles assembled within them,” Gatlin says. “This observation allowed us to rule out several competing hypotheses as to how scaling is achieved and, ultimately, decide on one.”
Publishing prestige
This marks the first time Gatlin has contributed a paper that has been published in Science, he says.
“If I wanted to be published in journals, Nature and Science would be at the top of the list,” says Gatlin, who started at UW in 2010. “In my field, Cell would be next.”
Oakey has been published in Science once before, in 2002, when he was a graduate student, he says. He was visibly excited about this paper’s findings.
“There is no doubt this paper is going to have a big impact on the field,” Oakey says.
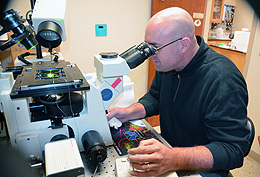
Photo:
Using microfluidics technology, Jay Gatlin, a UW assistant professor of molecular
biology, observes a mitotic spindle within a cell-sized droplet of cytoplasm through
a microscope. Gatlin and John Oakey, a UW assistant professor of molecular biology,
collaborated on research that is published in this month’s issue of Science.
Watch cytoplasmic droplets moving through a microfluidic device:
