- The Ouchterlony plates are
already poured and the wells have been punched out. In order to suck the plugs from the
wells, attach a Pasteur pipet to a vacuum flask. Carefully suck the plugs from the
wells. Take caution to only
remove agarose from the wells and not from the surrounding area.
2. From the side bench, collect one
tube containing antigen, (bovine serum albumin (BSA)) diluted 1:2. Collect a
second tube containing antibody to BSA (a-BSA).
Finally, collect a third tube containing sterile saline.
3. Prepare a
serial dilution of antigen (BSA). Collect 6 Eppendorf (eppi or microcentrifuge) tubes and label them #1
through #6.
a. Dispense 50 mL of saline (0.85%
NaCl) into each of the 6 tubes labeled #1 - #6. Use a P200 pipetman. It is
not necessary to change pipet tips between tubes as the solution is being
dispensed into sterile, empty microcentrifuge tubes.
b. Transfer 50 mL of antigen to
tube #1. Draw the solution up and
down gently 4-5 times in order to mix the solution and rinse the pipet. Do this gently to avoid making
bubbles!!!!
c. Using a new pipet tip, transfer 50 mL
from tube #1 to tube #2. Mix as in
step b.
d. Using another new pipet tip, transfer 50 mL from tube #2 to tube #3. Mix. Proceed the same way until the last tube
is mixed. Discard pipet tips
appropriately.
d. This series of two-fold dilutions
will generate the following:
Tube: #1 #2 #3 #4 #5 #6
Dilution: 1/4 1/8 1/16 1/32 1/64 1/128
- Label
the bottom of an Ouchterlony plate; use the diagram below.
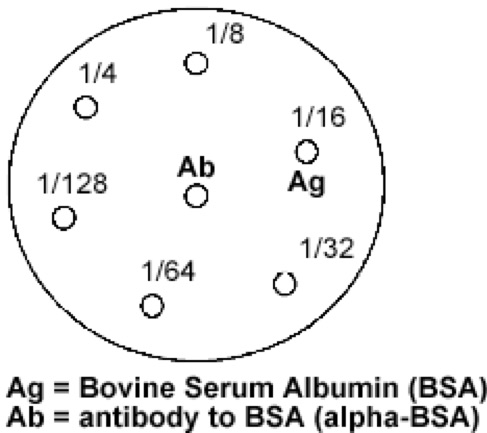
- With a P20 pipetman, dispense 10 mL of the diluted antigen into the outer
wells as labeled. If you start
with the 1/128 dilution and continue to the 1/4 dilution you can use the
same tip for all the antibody samples.
5. With a clean pipet tip, fill the
center well with 10 mL of antibody (a-BSA).
6. Be careful not to disturb the fluid
in the wells. Incubate, right side
up, for 48 hours at room temperature in a humidified chamber (Tupperware with
moistened paper towels in the bottom). |