Gram-positive unknowns: Culture A
_____ - Describe the growth of unknown A in broth culture. Describe the growth patterns both before and after mixing! Useful terms: Uniform fine turbidity (turbid throughout), flocculent (clumpy), pellicle (surface membrane), sediment, and ring (like a milk ring).
_____ - Take the nitrate broth tube to the nitrate test station and add the appropriate reagents. (Nitrate Results Procedure)
_____ - Describe the hemolysis (if any) on the Blood Agar plate. (BAP Examples)
_____ - Describe motility. This can be tricky; remember that if the entire tube looks turbid, the organism is most likely motile. If, by contrast, the growth is confined only to the stab line, the organism is likely nonmotile.
(Motility Examples)
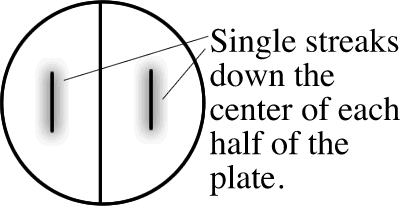
_____ - If unknown A is a Gram-positive rod, make 1 streakline on a split plate of Starch and Spirit Blue Agars. Use an inoculating loop and make only a single streak down the center of each half of the plate:
_____ - If your unknown is a Gram-positive and catalase positive coccus, streak one Mannitol Salt plate.
_____ - If your unknown is a Gram-positive and catalase positive coccus, perform a coagulase test. (Coagulase Test Procedure)
_____ - For Gram-positive and catalase negative cocci, streak a slant of bile esculin agar.
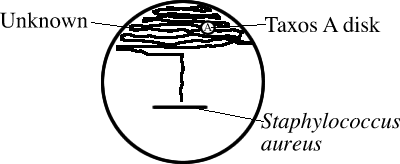
_____ - For Gram-positive and catalase negative cocci, perform a CAMP test and test for Taxos A (Bacitracin) sensitivity on the same Blood Agar plate (see sketch):
Gram-negative unknowns: Culture B
_____ � Describe the growth of unknown B in nutrient broth. Describe the growth both before and after mixing.
_____ � Describe motility.
_____ � Perform the nitrate test at the nitrate test station.
_____ � Inoculate one tube of urea broth using a loop.
_____ � Inoculate one tube of SIM medium using an inoculating needle to make a single stab to the bottom of the tube.
_____ � Inoculate one slant (Basic Slant Inoculation Procedure) of Simmons Citrate Agar using a loop to streak the entire surface of the slant.
After inoculation, place all the media into an incubation cup or rack.
As the coagulase and urease tubes are very small, it is sometimes helpful to tape them to the inside of the incubation cup. Place the cup / rack in the tray on the side bench to be incubated at 37�C. Be certain to place your working stock into the appropriate rack � DO NOT INCUBATE! If your Gram-positive unknown is a rod, place your MnCl2 slant into the rack with the working stock. This also needs to be stored in the refrigerator. The MR-VP tubes will remain in the incubator until the next lab period.


