Research Projects

Our research addresses two separate areas, one that involves alternative energy and the second involving carbohydrate modifications. Our research in alternative fuels centers on the design of new metal ligands for the photocatalytic splitting of water for hydrogen production. We focus on the use of rhenium metal and pyridine based ligands in our studies.
In the area of carbohydrates, we develop new metal-catalyzed methodologies for the transformation of carbohydates into new materials. The link below further elaborates the projects in both these areas.
Alternative Energy
The twofold problem of energy supply and global warming from an environmental, social and national security perspective have been manifested by the depletion of fossil fuels, their obvious impact on the environment and our nations lack of an crude oil supply. It is our group’s belief that the solar powered production of hydrogen from water is a viable and long term solution for this problem.
Recently, we have examined the photochemistry of the complex fac-Re(bpy)(CO)3H (bpy is 2,2'-bipyridine) and have found that visible light irradiation in THF produces H2 and the green dimeric species [fac-Re(bpy)(CO)3]2. This discovery led us to begin designing and testing the efficacy of binuclear complexes as catalysts for hydrogen production, and is a putative example of a chromophore and potential catalyst in the same structure. Thus, we design ligand such as the thiophene below (left) and complex rhenium to the bipyridines to produce the binuclear complex on the right. These substrates are then tested for their water splitting potential.
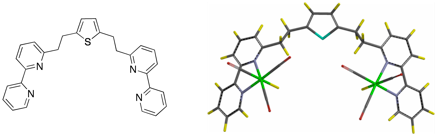
Synthesis of the complexes is illustrated below with substituted dipyridal ligands. This reaction scheme is representative of the standard sequence used and illustrates our involvement in both organic and inorganic synthesis.

“Facile Synthesis and Platinum complexes of 4’,5’,5’’-Trisubstituted-2,3’:6’,2’’-terpyridines” Huo, J.; Arulsamy, A. and Hoberg, J. O. Dalton Trans. 2011, 40, 7534-7540.
“A Facile and Inexpensive Synthesis of 6-Ethynylbipyridine” Huo, J. and Hoberg, J. O. I. J. Org. Chem. 2011, 1, 37-40.
Carbohydrate Methodology
We have developed methodology for the cyclopropanation and ring-expansion of glycals, which lead to the formation of seven-membered rings. Seven-membered rings are incorporated into a variety of biologically active natural products such as the complex cyclotoxins to simple mono-cyclic systems such as zoapatanol. This type of methodology thus provides for a convenient access into this area of biologically active compounds. Elaboration of the olefin moiety has also been achieved and we are able to make a variety of heptanoses (seven-membered ring sugars) by stereoselective addition reactions.
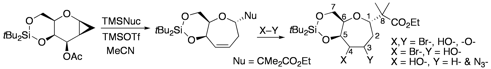
Most recently we developed methodology for the formation of vinyl lactones that can be converted to esters in good overall yields by treatment with catalytic amounts of palladium in the presence of nucleophiles.
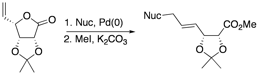
“Mechanistic studies of rearrangements during the ring-expansions of cyclopropanated carbohydrates.” Batchelor, R.; Harvey, J. E.; Teesdale-Spittle, P.; Hoberg, J. O. Tetrahedron. Lett. 2009, 50, 7283-7285.
“Heptosides from Galactose based Oxepanes via Stereoselective Addition Reactions.” Batchelor, R.; Harvey, J. E.; Northcote, P. T; Teesdale-Spittle, P.; Hoberg, J. O. J. Org. Chem. 2009, 74, 7627-7632. Featured Article
“Synthesis of 2,3-Dihydroxyl-4-hexenoates by Palladium-Catalyzed Allylic Alkylations of Carbohydrate Derived Vinyl Lactones.” Singleton, J.; Sahteli, K.; Hoberg, J. O. Synthesis, 2008, 3682.
“Synthesis of 2,3,4,6-tetra-O-benzyl-D-glucal on the gram scale. A convenient method for its facile synthesis and subsequent stereoselective cyclopropanation” Storkey, C. M.; Win, A. L.; Hoberg, J. O. Carbohydr. Res. 2004, 339, 897.
“Diastereoselective Formation of Seven-Membered Oxacycles by Ring-Expansion of Cyclopropanated Galactal” Batchelor, R.; Hoberg, J. O. Tetrahedron Lett. 2003, 44, 9043.
“Synthesis and Chemistry of Cyclopropanated Carbohydrates” Cousins, G. S.; Hoberg, J. O. Chem. Soc. Rev. 2000, 29, 165.
The development of inositols as C2-symmetric molecules for use in asymmetric synthesis is also of interest in our group. Inositols are a family of nine hydroxylated cyclohexanes that include the enantiomeric pair D- and L-chiro inositol. These are derived from the naturally occurring pinitol and quebrachitol, which are obtained from pine and rubber trees, respectively, by simple demethylation.
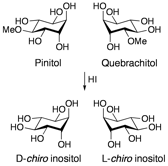
Our initial efforts have been in the design of chiral auxiliaries, in which the chiro-inositol is protected in two high yielding, selective steps to give alcohol 1. The alcohol is then esterified to give 2, treated with a nucleophile and selectively cleaved to give chiral acids in >99:1 ee. The auxiliary 1 is regenerated in the cleavage step and the crude material recycled to 2 in >80% yield by treatment with cinnamic chloride. Most recently we used these inositols as chiral ligands for asymmetric catalysis.
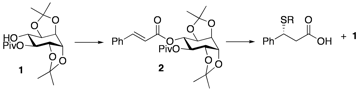
“Chiro-Inositols in Organic Synthesis” Singleton, J. and Hoberg, J. O. Mini-Rev. Org. Syn. 2009, 6, 1-8.
“Monoesterification of di-O-isopropylidene and di-O-cyclohexylidene chiro-Inositols” Cousins, G.; Falshaw, A.; Hoberg, J.O. Carbohydr. Res. 2003, 338, 995.
“Inositols as Chiral Templates: 1,4-Conjugate addition to tethered cinnamic esters” Cousins, G.; Falshaw, A.; Hoberg, J.O. Org. Biomol. Chem. 2004, 2, 2272.
Recent past work
Synthesis of Bioactive Compounds
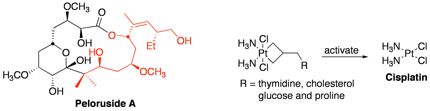
Peloruside A is a potent antimitotic macrolide isolated from the Pacific sponge Mycale hentscheli with cytotoxicity to murine leukemic cell lines at 18 nM. We have been active in the total synthesis of this macrolide in which our synthesis involves a variety of aldol reactions. We recently published methodology for the synthesis of the side-chain and its coupling to the pyranose portion (red portion below).
Additional work in the field of anti-cancer molecules involves synthesis of prodrugs of the anti-cancer molecule cisplatin. Our work focuses on the construction of inactive platinum(IV) complexes in the form of platinacyclobutanes, which are attached to cancer directing groups. Activation of the complex leads to cisplatin and a benign biomolecule.
“Studies on the Origin of 1,5-anti Induction in Boron-Mediated Aldol Reactions”
Stocker, B.L.; Teesdale-Spittle, P.; Hoberg, J. O. Eur. J. Org. Chem. 2004, 330.
“Synthesis of platinacyclobutanes bearing biological components for targeted, cisplatin prodrugs” Stocker, B.L.; Hoberg, J. O. Organometallics 2006, 25, 4537.