Solar energy is the only option for producing the vast quantities of renewable carbon-free power needed to power the planet. The Achilles Heel of solar energy is that the sun does not shine at night and so a method to store the energy for night and transportation uses is needed. Producing hydrogen from sunlight and water is an ideal solution to the storage problem. The Solar Hydrogen Activity research Kit (SHArK) Project at the University of Wyoming was established in 2008 as a spin off of our basic research effort on solar water splitting. This project provides a unique approach to learning chemistry that engages young people to participate in actual research to help solve the global energy problem.The goal of the SHArK Project is to discover metal oxide semiconductors that can split water into hydrogen and oxygen using sunlight. Currently, no known stable material is capable of efficiently and inexpensively photoelectrolyzing water with visible light. There are millions of compounds that need to be produced and tested for their ability to photoelectrolyze water. We developed inexpensive kits to enable young scientists to participate in this endeavor. The hardware and software was developed that uses an inkjet printer, laser pointer and LEGOs® allowing for virtually endless arrays of potential metal oxide semiconductors to be easily produced and tested.The SHArK project provides an avenue for researchers from all disciplines of science and engineering to collaborate to help solve the global energy crisis. In 2008, the Camille and Henry Dreyfus Foundation funded a seed proposal to create this project and currently 10 kits have been distributed to undergraduate institutions. SHArK is a branch of the Solar Army along with SEAL, HARPOON, and other projects. Currently, we are part of an NSF-funded project entitled "Powering the Planet" that focuses on solar water splitting (www.ccisolar.caltech.edu).
Unveiled in February 2016, SHArK 3.0 is currently undergoing beta-testing. This version of SHArK is faster and more reliable than ever! SHArK 3.0 also touts a cloud-based database of all SHArK results. When you save your results they will immediately be shared with all SHArK 3.0 users. Want to know when SHArK 3.0 is available for purchase? Join our mailing list. You can also email us at SHArK.Project@uwyo.edu, or simply check back here!
SHArK II
Originally, a Lego® Mindstorm kit was used to build a complex gearing system to tilt a pair of mirrors to raster a laser across the surface of the sample. Recently, SHArK has been upgraded to SHArK II. The replacement of the original back lash prone gears by the incorporation of Lego compatible linear actuators has increased the accuracy of the scanner and substantially reduced the time for a student to scan their sample from 2 hours to just 35 minutes. In addition, the data collection methods and user interface have been significantly enhanced to provide an instantaneous and more informative graphical representation of the photocurrent response.
A Hit, Published!
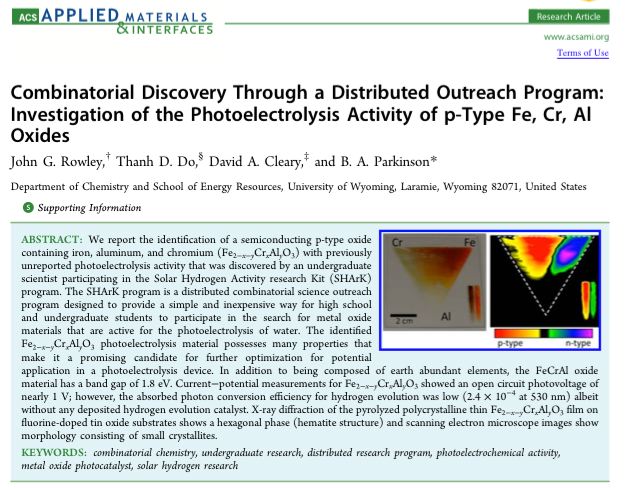
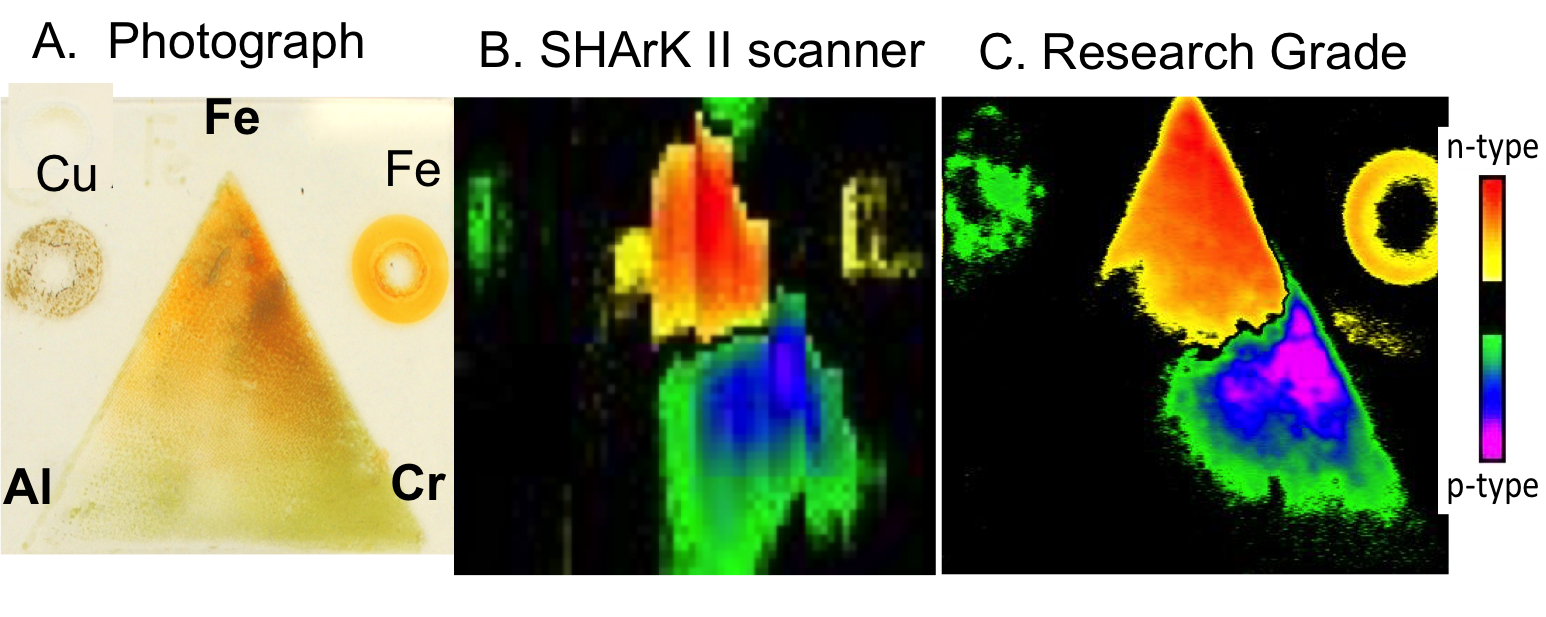
One promising p-type semiconductor, containing earth abundant iron, aluminum and chromium (Fe2-x-yCrxAlyO3), with previously unreported photoelectrolysis activity, was discovered by an undergraduate scientist at Gonzaga University participating in the Solar Hydrogen Activity research Kit (SHArK) program. The Parkinson group followed up on this “hit” and determined the near optimum stoichiometry to be Fe0.84Cr1.0Al0.16O3. The paper can be viewed here.
Silk Screening
Method

Silk screening was used to replicate the recently published ternary FexCrxAlx oxide photocatalyst. The results below show the n-type and p-type regions.