Alkane Dehydrogenation

An unsolved challenge in catalysis is the efficient and selective functionalization of simple alkanes and plentiful hydrocarbon feedstocks. One promising approach since the late 1990’s is the use of iridium with stable terdentate “pincer” ligands as alkane dehydrogenation catalysts:
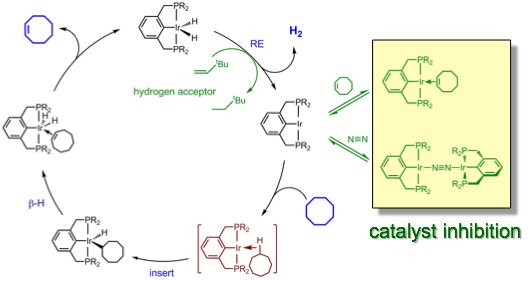
Calculations suggest that pincer ligands with electron-withdrawing phosphine substituents should have improved C-H addition kinetics. Accordingly, we developed the strong pi-acceptor pincer CF3PCPH and examined its iridium coordination chemistry (Adams, Organometallics 2011):
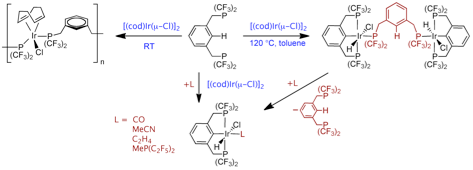
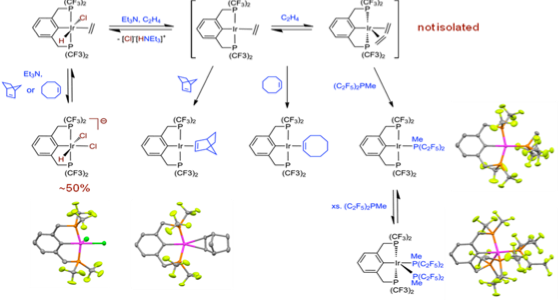
Dehydrohalogenation accesses a variety of four- as well as unusual five-coordinate Ir(I) systems which may serve as catalyst precursors (Adams, Organometallics 2011):
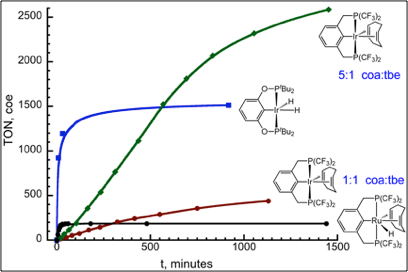
(CF3PCP)Ir(L) precursors (L = nbe, cod, dfmp, isoprene) have similar initial activities and convert to (CF3PCP)Ir(cod) as the catalyst resting state. Although the initial activity of these iridium catalysts is modest (40 TO hr-1) in 1:1 coa/tbe at 200 °C, high TON’s (~3000) and complete conversion of cyclooctane to cyclooctene is observed in 5:1 coa/tbe solutions. This catalyst is also quite insensitive to product inhibition by coe: in the presence of 800 equiv coe the initially activity is only reduced to 67%. Unlike donor phosphine pincer catalysts, the activity of (CF3PCP)Ir(cod) is unaffected by N2 or excess water.
Another distinctive feature of the PFAP pincer system is that the reaction of (CF3PCP)Ir(alkene) complexes with H2 do not afford (CF3PCP)Ir(H)x, but rather a novel binucleating pincer polyhydride product:
We have recently reported the first example of a group 8 alkane dehydrogenation catalyst, (CF3PCP)Ru(cod)H (Gruver, Organometallics 2011). Although this system has very limited catalyst stability, it represents a very exciting entry to new relatively inexpensive 4d metal catalysts and has considerable promise for further development.
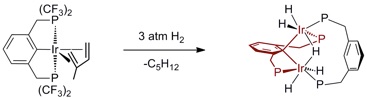
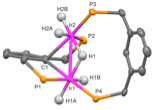
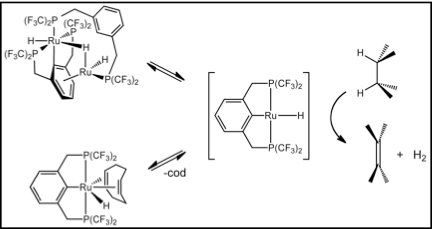
These PFAP pincer catalyst systems provide a starting point for the development of practical air- and moisture-stable alkane dehydrogenation systems
