Alkene Oligomerization

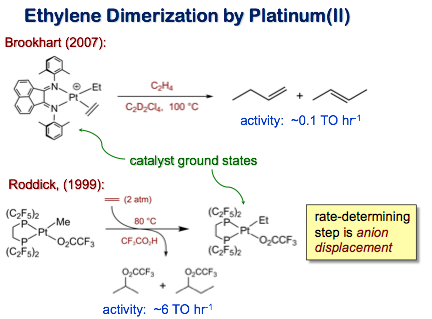
A major advance in alkene oligomerization catalysis was Brookhart’s 1995 report of highly active group 10 metal diimine systems. In contrast to nickel and palladium catalysts, the corresponding platinum diimine system has very limited activity towards ethylene dimerization. In 1999 we reported a related PFAP system which, though somewhat more active, was limited by slow anion dissociation (White & Bennett, Organometallics 1999):
We have more recently reported the synthesis of a labile platinum PFAP system which is considerably more active (Basu, Organmetallics 2008). Production of 2-butenes occurs at subambient temperatures and is followed by proton-mediated oligomerization initiated by highly acidic platinum hydride intermediates:
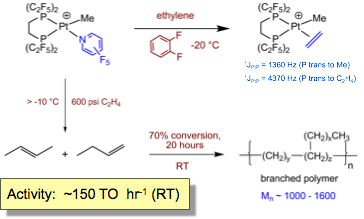
The dimerization activity of electron-poor (dfepe)Pt(Me)(C2H4)+ is orders of magnitude greater than the corresponding diimine system: subsequent kinetic studies have determined the ethylene insertion barrier for (dfepe)Pt(Et)(C2H4)+ to be 17.3 kcal mol-1, nearly 13 kcal mol-1 lower than that reported for the diimine analog (29.6 kcal mol-1) and comparable to palladium diimine catalysts (17 - 19 kcal mol-1)
Why are PFAP systems so much more reactive than conventional diimine analogs? DFT calculations as well as a current program of developing a wide range of alkylphosphine analogs indicate that lower alkene insertion barriers are due to a combination of high phosphine trans influence (i.e., a weakening of the trans metal-alkyl bond) and a reduction of metal electron density imparted by PFAP ligands. We are currently exploring these ideas with the aim of establishing a new class of highly-active oligomerization catalysts.