Perfluoroalkylphosphine Overview

Our research interests stem from a simple approach to ligand design that we began upon our arrival at the University of Wyoming in 1986. Despite the wide array of ligands (particularly phosphines) employed in coordination chemistry, the combination of strong pi-acceptor ability with significant steric bulk and tunability was essentially missing:
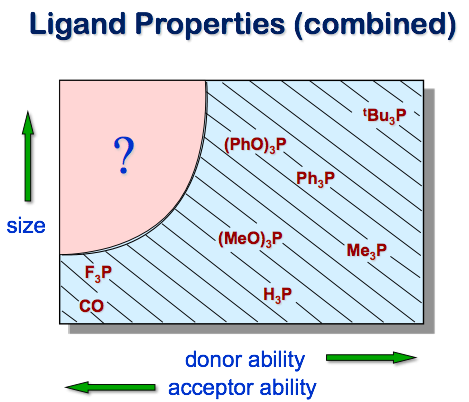
PF3 has a pi-accepting ability comparable to carbon monoxide, but does not provide an opportunity for steric tuning. The first perfluoroalkylphosphine (PFAP), (CF3)3P, was reported by Haszeldine in 1950, and the chelate (CF3)2PCH2CH2P(CF3)2 by Burg in 1961, but very few applications of this ligand class had been reported. We have expanded the range of PFAP ligands available for research using C2F5 substituents; Caffyn has more recently used TMSCF3 to efficiently substitute CF3 for P-OAr. Although fully perfluoroalkylated phosphines are available (and provide the highest level of pi-accepting ability), we have generally observed that one hydrocarbyl group is required for optimal metal binding.
