Titration of a Phage Suspension
- In a test tube rack, set up 1 glass tube (label it #1) and 5 small plastic dilution tubes (labeled #2-6).
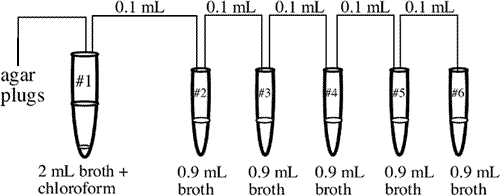
- Using a 1.0 mL pipet, transfer 2.0 mL of sterile broth into tube #1. Aliquot 0.9 mL of sterile broth into each of tubes #2-6. The procedure is below, you will need to do it 6 times to complete this step.
- Using a glass Pasteur pipet, add 4 drops of chloroform into tube #1. Now prepare a serialphagedilution from the sewage isolation done in the previous lab. Follow the instructions illustrated in the diagram below and described in steps 4 and 5.
- Place a rubber pipet bulb on a Pasteur pipet. Stick the tip of the pipet through an area of lysis until it touches the bottom of the plate. Withdraw the pipet at an acute angle. A small plug of agar should be seen in the tip of the pipet. Expel this plug into tube #1, which contains 2.0 mL of broth and chloroform. Repeat this 3 - 4 more times to make sure that tube #1 has phage in it. The chloroform in tube #1 will kill any bacteria picked up in the plug of agar.
- Mix tube #1 well by tapping. Wait for the chloroform to settle to the bottom of the tube.
- Remove 0.1 mL from tube #1 (avoid the chloroform at the bottom of the tube) and put this aliquot into tube #2. Mix tube #2 well and continue the serial dilution (below) out to tube #6.
- Label 3 LB agar plates with your initials and section number. Number the plates #4, #5 and #6.
- Take the plates and a rack with the phage dilutions (only Tubes #4, #5 and #6) to a 55˚C water bath.
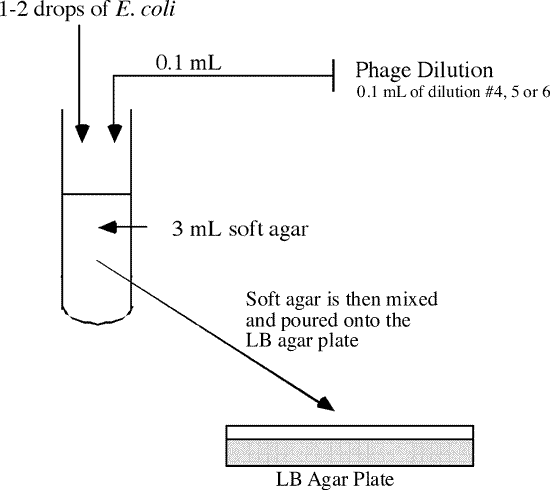
- Collect 3 sterile tubes, each containing 3 mL of soft agar, from the water bath.
- The next 2 steps must be done quickly to keep the soft agar from solidifying before it can be poured. See the diagram below for an illustration of the procedure.
- Remove one of the tubes containing the soft agar and add 1-2 drops of Escherichia coli culture. Then add 0.1 mL from phage dilution #4 into the same soft agar tube.
- DO NOT PLACE THE TUBE BACK INTO THE WATER BATH.
- Roll the tube between the palms of your hands quickly to mix, and pour the entire contents onto the LB agar plate labeled #4.
- Swirl the plate gently, but quickly to ensure complete coverage before the agar solidifies. This is the overlay technique.
- Repeat this procedure for phage dilution #5 and #6.
- After the agar has solidified (about 10 min) invert the plates and place them in the tray on the side bench to be incubated at 37˚ C.
|