Photoinduced Electron Transfer Reactions and Electron Transfer Sensitizers
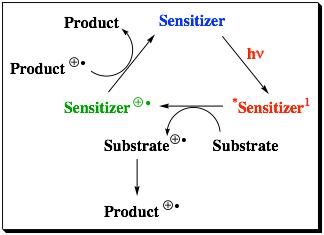
The explosion of interest in “photoinduced electron transfer” (PET) reactions can be readily demonstrated by searching for this term in the SciFinder Scholar database. The one manuscript located with this term published in the 1950s increases to 3 in the 1960s, 47 in the 1970s, 572 in the 1980s, 3259 in the 1990s, 4110 in the 2000s, and the increase in interest remains unabated since 2010. The remarkable interest in this topic reflects the importance of this process in biological systems and in photovoltaics, and the utility of PET to make new materials. Unfortunately, the low quantum yields of many photoinduced electron transfers provide a significant impediment to achieving an energy efficient environmentally green process. These low efficiencies can be attributed in many cases to energy wasting return electron transfer (RET) in the ion pair intermediates. A significant amount of effort has been expended to circumvent this detrimental RET with mixed results. One of the most successful methods to inhibit RET has been the use of charge-shift reactions (Eqn. 1). The success of this method has been attributed to the lack of an electrostatic interaction between the reduced sensitizer and oxidized substrate that allows diffusive separation, kSEP, to compete with RET. For example, an improvement of over 80% in the quantum yield of ion pair separation in the electron transfer to form biphenyl radical cation in CH3CN was reported by changing the sensitizer from 2,6,9,10-tetracyanoanthracene, which produces a radical-anion/radical-cation pair, to N-methylacridinium ion, which produces a radical/radical-cation pair lacking a Coulombic barrier to separation. Despite this impressive result the quantum yield only increased from 0.18 to 0.33 leaving much room for improvement. Consequently, a major goal in our previous grant period was to extend the charge shift concept by synthesis and characterization of dicationic sensitizers that would generate repulsive radical-cation/radical-cation pairs. (Eqn. 2)
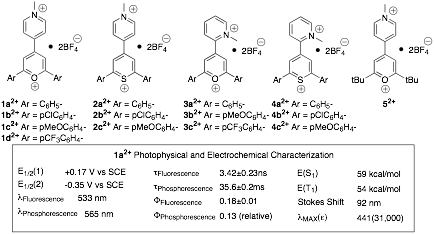
The dications which are under investigation in the group are pyrylogens (hybrids of viologens and pyrylium cations)
Polyaromatic Viologens
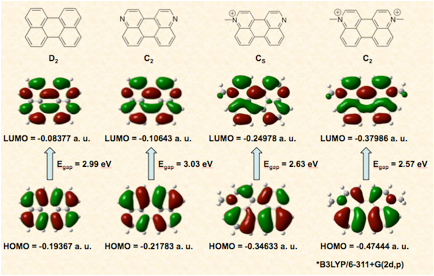
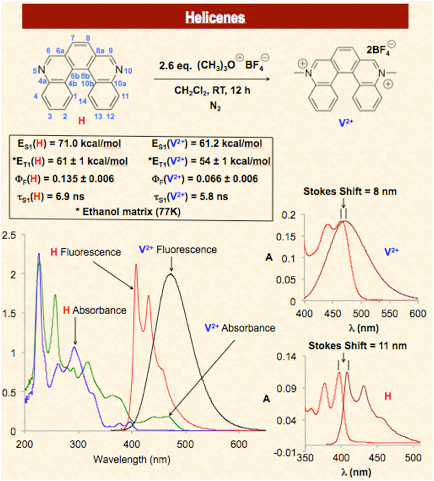
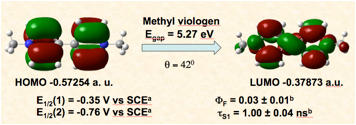
Viologens are the diquaternary salts of 4,4’-bipyridine. In recent years their applications have dramatically expanded from their early use as herbicides to their use as redox indicators, electron transfer relays, DNA photocleaving agents, and as components of charge transfer salts and supramolecular assemblies. Unfortunately, the prototypical viologen, N, N’-dimethyl-4,4’-bipyridine (methyl viologen) does not have a band gap or photophysical properties amenable to its use as a sensitizer or as an electronic material despite its attractive redox properties. The goal of this research is to expand the structural landscape of viologens by embedding its functional group in polyaromatic hydrocarbons (PAHs). The hope is that these new viologens will retain the well established materials properties of the PAH, including their value as components of molecular switches, field effect transistors, organic voltaic devices, and organic light emitting diodes (OLEDs), while simultaneously adding a new, attractive, and addressable redox feature to the PAH.
![]()