INtroduction to Johne's Disease
Johne's disease, caused by the bacterium Mycobacterium avium subspecies paratuberculosis (MAP) – also referred to as paratuberculosis – is a chronic, progressive, and ultimately fatal gastrointestinal disease that primarily affecting domestic ruminants (cattle, sheep, goats, camelids and buffaloes) and wild ruminants (such as deer, elk, and bighorn sheep). Currently there is no effective treatment or cure, and no commercially available vaccine in the United States. Although historically associated with dairy cattle, Johne's disease is becoming an increasing concern in beef herds [USDA, 1997; Johnson et al. 2022].
At the WSVL, we are launching a new state-wide prevalence study in cattle ("Understanding Johne's Disease in Wyoming Beef Cattle"), and are looking to include 100 beef herds across Wyoming, with 30 animals per herd. If you are interested, clink the link below for more information.
The Pathogen and Disease Progression
- Causative Agent: Mycobacterium avium subspecies paratuberculosis (MAP) is a hardy bacterium that can survive in the environment for extended periods (up to a year), though it only replicates within the host’s macrophages.
- Infection in Young Animals: Calves, lambs, and kids are most susceptible, typically acquiring the infection through ingestion of manure-contaminated feed, water, milk, or directly from infected dams. In utero transmission is also possible.
- Subclinical Phase: Infected animals remain clinically normal for months to years while MAP colonizes intestinal tissues.
- Clinical Signs: Clinical signs typically manifest in adult animals (usually >18 months) and is characterized by progressive weight loss despite a good appetite. In cattle, chronic diarrhea is common in later stages of disease. Submandibular edema ("bottle jaw") due to protein loss may also occur in advanced stages. In small ruminants, sheep tend to show clinical signs less frequently than goats, and diarrhea is less common in both. For instance, only 20% of infected sheep show diarrhea at the end stage, and goats often do not exhibit diarrhea at all.
- Transmission: Fecal-oral transmission from infected adults to youngstock is the primary route. Shedding of MAP in feces increases over time, and MAP may also be present in milk and colostrum. Introduction of silently infected animals into a herd is a major risk factor.
- Environmental Contamination: Manure (feces) is the main vehicle for environmental spread. MAP can persist in soil and water for extended periods, contributing to herd-level transmission risks. [Grant, 2010]
Rising Concern in Beef Cattle
Surveys and diagnostic data suggest an increasing prevalence of MAP infection in U.S. beef herds, particularly in purebred operations with frequent animal movement. The current prevalence in U.S. sheep and goat herds remains unknown (NAHMS, 2023).
Defining Your Testing Goals
Clarifying the objective of testing is essential before selecting a diagnostic approach. Consider whether the goal is to:
- Initially screen for MAP presence in the herd
- Estimate herd-level prevalence
- Control spread within a known infected herd
- Work toward eradication from the herd
- Diagnose individual cases
- Perform pre-purchase risk reduction
Your testing goal will guide the most appropriate type and scope of diagnostics.
Diagnostic Tools available
Our laboratory offers a suite of diagnostic options to support various management objectives for Johne’s disease. The choice of test depends on the specific context and goals of testing.
Organisim Detection Tests (Detect MAP directly)

- Principle: Detects MAP DNA (targeting IS900 sequence) in manure or tissue. PCR identifies both live and dead organisms.
- Turnaround Time: 1-7 days
- Sensitivity & Specificity:
- High sensitivity (~95%) for detecting moderate-to-heavy shedders
- Moderate sensitivity (~75%) for low-level shedders or early-stage infections
- Specificity near 100% (very low false-positive rate). (Cornell, accessed 2025-05-16)
- Applications:
- Herd Screening (Pooled PCR): Cost-effective in low-prevalence herds. Lab pools 5 manure samples per test; submit individual samples with animal ages to allow efficient pooling in descending age order (Kalis 2000).
- Individual Animal Diagnosis: Useful for clinical suspect animals.
- Post-mortem Diagnosis: PCR can be run on intestinal contents.
- Culture Confirmation: PCR can confirm that MAP was cultured (although WSVL does not offer culture – see below).
Due to MAP’s extremely slow growth – colonies may take 6-12 weeks to appear and up to 6 months for full incubation – WSVL does not offer culture testing for MAP.

Antibody Detection Tests (Detect immune response)
- Principle: Detects MAP-specific antibodies in serum. Antibody levels increase in later stages of infection.
- Turnaround Time: 1-3 business days
- Sensitivity & Specificity:
- Specificity: High (97-99%), low false-positive rate
- Sensitivity: Lower in early infection dues to delayed antibody response (Nielsen & Toft, 2008)
- Applications:
- Herd Screening: Especially cost-effective for large herds.
- Identifying Potential Shedders: Higher antibody levels are often associated with active shedding (later stages of infection).
- Pre-purchase Testing (Buyer Perspective): While a negative ELISA does not guarantee freedom from infection, it lowers risk. However, a positive ELISA in an animals from a low-prevalence herd may represent a false positive and should be interpreted with caution.
- Sample Submission: 2-3 mL of blood in a red-top tube (or serum separator tube). Discounted pricing is available when more than five samples from the same herd are submitted together (see our Fee Schedule).
Post Mortem (Necropsy)
Necropsy remains an important diagnostic tool in identifying Johne’s disease, especially in animals with chronic weight loss or other compatible clinical signs. Post mortem examination allows direct visualization and sampling of characteristic lesions in the ileum and associated lymph nodes.
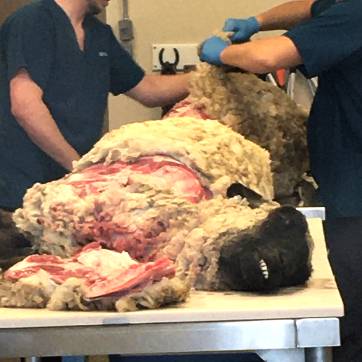
- Who to Submit: Animals >18 months old with a history of progressive weight loss, with or without diarrhea, are the most informative candidates.
- Gross Lesions: Thickening of the terminal ileum and enlarged mesenteric lymph nodes may be observed in advanced cases.
- Diagnostic Testing: PCR testing can be performed on intestinal contents or affected tissues to confirm MAP presence. Histopathology of formalin-fixed tissues (ileum and ileocecal lymph nodes) is also useful to detect granulomatous inflammation consistent with Johne’s disease.
- General Value: Beyond Johne’s disease, necropsy can help identify other causes of wasting and chronic illness, making it a valuable part of herd health investigation.
Understanding the Impact of Herd Prevalence
The prevalence of Johne’s disease in a herd significantly affects the interpretation and reliability of diagnostic results:
- Low Prevalence Herds: In herds with a low estimated prevalence, a positive ELISA result is more likely to be a false positive, while a negative result is more likely to be accurate. In these herds, more sensitive tests like PCR may be preferred for initial screening.
- High Prevalence Herds: In herds with higher prevalence, a positive ELISA result is more likely to reflect true infection, but negative results may miss infected animals that have not yet mounted a detectable antibody levels response.
- Unknown Prevalence: When prevalence is unknown, test results should be interpreted cautiously. Follow-up testing with a different method (e.g., PCR confirmation of ELISA-positive animals) is advised.
WSVL Tests for Your Needs (WOAH, 2025)

Important Considerations for Testing
- Test Adult Animals (≥ 18 months): Diagnostic tests are most reliable in adult cattle, sheep and goats, as they are more likely to be shedding MAP or have detectable antibodies.
- Customized Testing Strategies: Tailor your testing approach to align with your herd’s specific goals, risk factors, and management conditions.
- Proper Sample Handling: Follow lab guidelines for sample collection and packaging to prevent contamination and ensure test accuracy.
Control and Prevention Strategies: Integrating Diagnostics
Diagnostic testing is a cornerstone of Johne's disease control and prevention. Since no effective treatment exists, identifying infected animals is key to limiting the spread of MAP – particularly to young, susceptible calves, lambs, and kids. The timeframe for achieving control depends on herd prevalence and the chosen strategy.
Key Control Measures

Conduct annual whole-herd or targeted testing (via ELISA or PCR) of adult animals to identify and remove MAP shedders. Shedding often increases during stress (e.g. calving/lambing, extreme weather, feed changes, illness). Testing before the calving/lambing season is strongly recommended.
Offspring of MAP-positive dams are at higher risk of infection. Consider culling them or, if retained, do not use them as replacements.
MAP can survive in the environment for up to a year. To limit environmental transmission:
- Protect feed and water sources from manure contamination. Fence off contaminated ponds and provide clean drinking water.
- Maintain clean calving areas and avoid overcrowding. Move pregnant cows to fresh calving pastures and promptly segregate calves by age (Sandhills Calving System principles, and strategies to prevent neonatal calf diarrhea [Pence et al, 2001; Radostits et al 1983; Thomson, 1997]). Similar management strategies are effective for ewes and does.
- Manage traffic areas by placing mineral feeders and hay away from high-traffic calf zones to reduce manure buildup.
- Rotate pastures to help reduce MAP persistence.
- Source animals from herds with documented negative Johne’s status based on whole-herd testing.
- Avoid introducing manure, colostrum, or mild from unknown-status herds.
- Quarantine new additions and consider repeated testing over time, especially if sourced from high-risk herds.
- Know the status of the source herd – this is more reliable than testing a single animal.
Prevention: The Most Cost-Effective Approach
Preventing the introduction of Johne's disease is far more economical than managing an existing infection. When purchasing animals:
- Prioritize those from test-negative herds with a documented health history.
- Be cautious of animals from herds with unknown or unverified Johne’s status.
|
Test Strategy Checklist To develop a strategic testing plan, consider the following:
|
References
- USDA : APHIS (United States Department of Agriculture : Animal and Plant Health Inspection Service). (1999). Info Sheet: Veterinary Services. What do I need to know about Johne’s disease in beef cattle? Centers for Epidemiology and Animal Health. Online: https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy96/Dairy96_dr_Johnes.pdf. Accessed: May 19, 2025
- USDA : APHIS (United States Department of Agriculture : Animal and Plant Health Inspection Service). (2010). Uniform Program Standards for the Voluntary Bovine Johne’s Disease Control Program (APHIS 91–45–014).
- USDA : APHIS (United States Department of Agriculture : Animal and Plant Health Inspection Service). (2024). NVAP Reference Guide: Johne’s Disease (Control and Eradication). Online: https://www.aphis.usda.gov/print/pdf/node/1944. Accessed: May 19, 2025
- Johnson P, Marfleet T, Waldner C, Parker S, Campbell J. (2022). Seroprevalence of Mycobacterium avium spp. paratuberculosis in cow-calf herds located in the prairie provinces of Canada. Canadian Veterinary Journal, 63(12), 1247. PMID: 36467383
- Grant IR. (2022). Mycobacterium avium subsp. paratuberculosis in animal-derived foods and the environment (29-39). In Paratuberculosis - Organism, Disease, Control. M Behr and D Collins Eds. United Kingdom: CAB International. DOI:10.1079/9781845936136.0029
- USAHA (United States Animal Health Association). (2023). Resolution #35. Johne’s Disease Prevalence - National Animal Health Monitoring System 2024. 127th Annual Meeting - October 12-18, 2023. Online: https://usaha.org/usaha-resolutions/. Accessed: May 19, 2025
- Cornell – AHDC. (2025). Online: https://www.vet.cornell.edu/animal-health-diagnostic-center/testing/testing-protocols-interpretations/johnes-direct-fecal-pcr-test. Accessed: May 16, 2025
- Nielsen SS, Toft N. (2008). Ante mortem diagnosis of paratuberculosis: A review of accuracies of ELISA, interferon-γ assay and faecal culture techniques. Veterinary microbiology, 129(3-4), 217-235. DOI: 10.1016/j.vetmic.2007.12.011
- Ly A, Sergeant ES, Plain KM, Marsh I, Dhand NK. (2021). Simulation modelling to estimate the herd-sensitivity of various pool sizes to test beef herds for Johne's disease in Australia. Preventive Veterinary Medicine, 189, 105294. DOI: 10.1016/j.prevetmed.2021.105294
- WOAH (World Organisation for Animal Health). (2025). Manual of diagnostic tests and vaccines for terrestrial animals. Section 3.1.16. Paratuberculosis. Accessed May 15, 2025
- Johnson P, McLeod L, Campbell J, Rousseau M, Larson K, Waldner C. (2022). Estimating the sensitivity and specificity of serum ELISA and pooled and individual fecal PCR for detecting Mycobacterium avium subspecies paratuberculosis in Canadian cow-calf herds using Bayesian latent class models. Frontiers in Veterinary Science, 9, 937141. DOI: 10.3389/fvets.2022.1003143
- Sandhill Calving System (https://beef.unl.edu/beefreports/symp-2007-17-xx.shtml)
- Pence M, Robbe S, Thomson J. (2001). Reducing the incidence of neonatal calf diarrhea through evidence-based management. Compendium on Continuing Education for the Practicing Veterinarian 23:S73-S75
- Radostits OM, Acres SD. (1983). The control of acute undifferentiated diarrhea of newborn beef calves. Veterinary Clinics of North America. Large Animal Practice, 5(1):143-55
- Thompson JU. (1997). Implementing biosecurity in beef and dairy herds. In American Association of Bovine Practitioners Conference Proceedings 30: 8-14

