NMR Useful Tips
NMR Facility
Lock issues
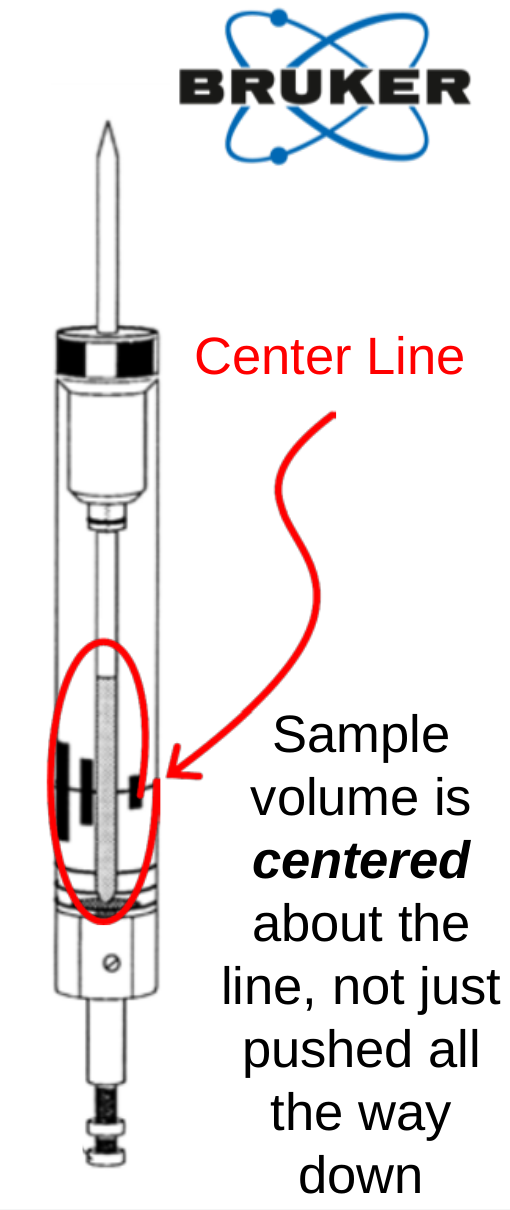
If the sample does not lock, please make sure that you followed the "NMR-basic-operation" procedure, in particular, make sure that you followed the procedure about sample preparation. Do you have enough liquid, did you really add deuterated solvent, and is the sample placed within the gauge correctly, did you read an appropriate shim file with 'rsh'?
If the sample still does not lock, please do the following:
Type 'ii' <enter>. If there is an error, repeat.
Try 'lock' again.
Try manual locking (see below).
If the sample still does not lock, you may try:
Manual locking:
Make sure that you have the BSMS Control window open. If not, type 'bsmsdisp'. Please go to the LOCK tab.
Click on "Auto LOCK" and wait for about 1 minute. If it locks, fine, if it doesn't, please continue with manual locking.
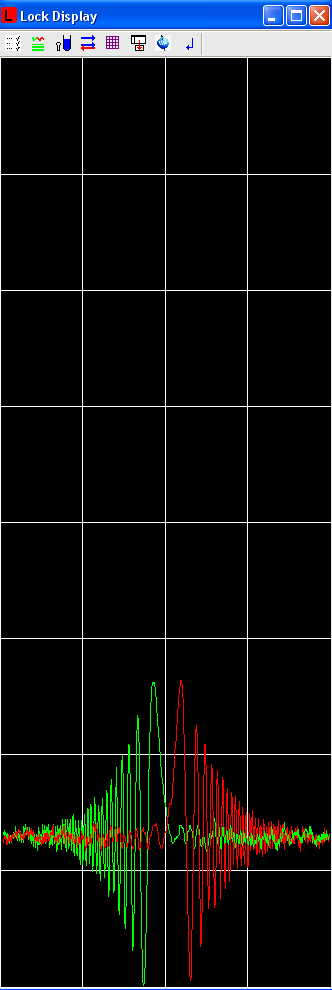
The goal of locking is to have a lock signal displayed that is symmetrical and centered, not too small or too big, with significant ringing. Probably you have observed this kind of image many times for many successfully locked samples.
If the lock signal shows no signal, or is not centered: BSMS Control Suite/Lock -> Select: Field -> Adjust Field
If the lock signal is too big or small: BSMS Control Suite/Lock -> Select: Lock gain -> Adjust gain. (Optional: Power, but be careful because of power saturation effects)
If the lock signal is not symmetrical: BSMS Control Suite/Lock -> Select: Phase -> Adjust phase.
When you adjust the values within the BSMS Control Suite, please make sure that the cursor does not leave that window. It may be convenient to adjust the stepsize.
If everything is adjusted, push: 'On' within the BSMS Control suite / Lock, and it should lock.
The spectrum may be shifted later. If so, it is possible to calibrated the axis by typing 'cal', which opens a dialog box. Manual calibration is an interactive tool to set the x-axis to the appropriate chemical with known chemical shift (e.g., a solvent).
Shimming Issues
After performing automated shimming with "topshim".
.if it says: "not enough valid points', make sure you read the default shim file with 'rsh'.
.if it says: "too many points lost during fit", try: 'topshim convcomp' to compensate for convection currents, especially for non-viscous solvents that are prone to convection currents.
It is possible to do shimming without locking. To do so, read the next section.
Shimming non-deuterated solvents
While it is easiest to have deuterated solvents as it makes shimming and long-term experiment more stable, it is not essential. Shimming is still recommended and can be done in various ways.
1. Run a 1-D simple proton experiment with the default shim set (type: rsh <enter> and select an appropriate shim file, see also the basic operation instructions) and take a note of the location (in ppm) of a prominent line.
2. gs <enter> runs the experiment in "live" mode, so you can watch the spectrum change while you change the shims at the same time. To do this, open the BSMS window (type bsmdisp <enter>). Turn the LOCK OFF on the BSMS, and press on SHIM.Modify the shims to maximize the proton amplitude and the shape of the signal.
3. Automatic shimming on a proton line.
The command topshim lockoff 1h o1p=x.xx
will turn the lock off, set the proton frequency for x.xx ppm
If this does not work, return to manual shimming on step 2.
Acquisition Problems due to breakdown of communication between console and computer
These problems manifest themselves quite often and there is no harm in following below instructions.
ii<enter>. If there is an error, repeat.
ii restart <enter>. If there is an error, repeat.
Variable temperature NMR experiments using the liquid nitrogen evaporator
Precautions:
- Best to use for temperatures below 0 °C. For temperatures between 0 °C and 25 °C (room temperatures), it is best not to use liquid nitrogen as the cooling gas, as the sample heater must be used at high current with the increased risk of heater burn-out and the temperature stability may be poor.
- Make sure that the sample solvent stays liquid at all temperatures.
- Never conduct high temperature experiments in a sealed tube or near the boiling point of the solvent.
- Solubility may be reduced at lower temperatures.
- Calibration is necessary to know the exact temperature. That can be done with a solution of 4 % methanol in methanol-d4 for low temperature. For high temperature, a solution of 80 % ethylene glycol in DMSO-d6 may be used.
- Allow ~ 10 minutes between each change of 10 Degrees, both cooling down, and coming back to room temperature.
- Use the ceramic spinners (white) for all variable temperature experiments. Do not use the plastic spinner above above ~ 30 °C.
- The upper limit on heating is 150 °C, the lower limit for cooling is -150 °C.
- Always bring back the temperature to room temperature and turn off the heater when you are finished.
Procedure:
- Fill the low temperature NMR dewar. It is heavy when it is full, make sure you do not use a metallic cart when transporting it near the magnet.
- Attach the liquid nitrogen evaporator connector to the TC-2T connector.
- Slowly lower the heat exchanger into the liquid nitrogen dewar and secure in place with clamp. A full dewar may last about 10 hours, depending on the temperature desired.
- Move the dewar into place and put the hose in position to attach to the probe.
- Remove the gas line from the probe.
- Attach probe to the coupler on the heat exchanger.
- In TOPSPIN, edte <enter> opens the temperature settings window. Turn on both HEATER and COOLING. Set the temperature to +15 °C, flow rate = 500 L/h, set max of heater = 10 % (the lower the better for temperature stability, do not put values above 50 %). Better not to change temperatures faster than 10°C/10 minutes. You may need to adjust the cooling power which controls the current to the nitrogen boil-off heater. That can be changed by clicking on CHANGE next to the cooling window. More current generates more cold gas and allows a lower temperature to be reached. However, too much cold gas will lift the sample out of the probe.
- Lock, shim, tune and match may change when you change temperatures. It is best to lock and shim at room temperature. At lower temperatures, locking may be more difficult. It is best to tune and match the probe at each new temperature.
- The "shim coil temperature", visible in the main window of TOPSPIN, needs to be in the proper temperature range. There is a warning if it drops below +5 °C. It should not be allowed to go below freezing. This can be done by adjusting the "SHIM flow". This is done automatically on the 600 MHz NMR spectrometer or 300 MHz NMR spectrometer, but needs to be manually adjusted with a knob on the 400 MHz NMR spectrometer at the "SHIM flow" line. Turn on the flow just slightly. It is best to avoid turning on the flow too much, otherwise temperature control may be difficult.
- Change temperature no faster than 10°C/10 minutes.
- When you are finished with the experiment, bring back the temperature to room temperature.
- Turn off both the sample heater and nitrogen boil-off heater.
- Make sure that the coupler of the heat exchanger to the probe is thawed before you attempt to remove it from the probe.
- Remove heat exchanger from probe.
- Attach the gas line to the probe.
- If you adjusted the "SHIM flow" with the knob (on the 400 MHz NMR spectrometer), don't forget to turn it off again.
- Put away the liquid nitrogen evaporator.
Near room temperature variable temperatures or alternative variable temperature procedure
For temperatures between 0 °C and 25 °C (room temperatures), it is best not to use liquid nitrogen as the cooling gas, as the sample heater must be used at high current with the increased risk of heater burn-out and the temperature stability may be poor.
Instead, it is better to let the air that goes to the NMR probe pass through a coil of copper tubing that is immersed in cold solvent such as a water ice, dry ice/solvent or salt/ice cooling bath. Be careful not to spill the contents.
Popular cooling baths include:
| Cooling Bath | Temperature in °C |
| Dry Ice-Benzene | 5 |
| Ice Water | 0 |
| Ice-Acetone (1:1) | -15 |
| Ice-NaCl (1:3) | -20 |
| Dry Ice-Acetone | -78 |
| Liquid Nitrogen | -196 |
| Liquid Helium | -269 |
It is possible to reach other temperatures by using the heater in the edte-window. The heater power should stay low for temperature stability and safety issues (burn-out), so best choose cooling baths close to the desired temperature.
NMR software
Topspin now free for Academic Users (PC, Mac, Linux)
This software is the standard software that is used to acquire data from the Bruker spectrometers and should be familiar to the Bruker NMR users. Bruker has made the Topspin software free for academic users. Please read details at https://www.bruker.com/products/mr/nmr/nmr-software/nmr-software/topspin-faqs.html
NUTS (PC, Mac, Linux via Wine)
The University has a license for this software, details are at: http://www.acornnmr.com/ and at: instrumentation/NMR/60-nmr-basic-operation
MESTRE-NOVA (Mnova) (PC, Mac, Linux)
This software can process both NMR and MS dfata. Details are at: http://www.mestrelab.com/
ACD (PC)
Details are at: http://www.acdlabs.com/
Spinworks (PC)
Free software for processing of 1D and 2D spectra. Details are at: http://www.umanitoba.ca/chemistry/nmr/spinworks/
iNMR (Mac)
Details are at: http://www.inmr.net/
To import Bruker software raw data and processed data, access the following files:
fid (for 1D data)
ser (for 2D data)
1r (for 1D data processed data within the folder pdata/
2r (for 2D data processed data within the folder pdata/
Page created by Alexander Goroncy.