Grasshoppers of Wyoming and the West
Entomology
Devastating Grasshopper
Melanoplus devastator (Scudder)
 |
Distribution and Habitat
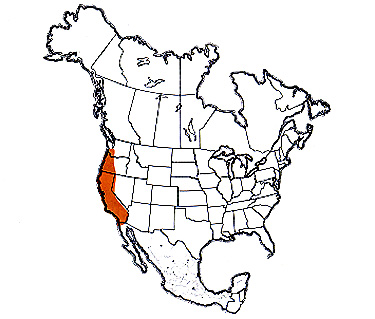
The devastating grasshopper has a limited geographic range in the far west of North America. Its main distribution is in the coastal states, where it inhabits semiarid rangelands dominated by forbs and annual grasses at elevations from near sea level to over 5,000 feet.
Economic Importance
The devastating grasshopper, a major pest in California and a minor one in Oregon and Washington, destroys rangeland forage, orchards, grains, vegetable crops, and gardens. Populations, ever present on rangeland in the coastal and Sierra Nevada foothills of California, fluctuate annually in size. Significant damage to rangeland occurs when densities rise to outbreak levels.
During a prolonged outbreak, more than 3 and 4.5 million acres of rangeland were infested in 1957 and 1958, respectively. Although several species were involved, the devastating grasshopper generally predominated in the assemblages. Forage of the infested rangeland, often harboring from 35 to 100 devastating grasshopper late nymphs and adults per square yard, became exhausted. The grasshoppers then moved to the valleys where green pastures, crops, and gardens flourished. The hungry marauders defoliated orchards and vineyards and destroyed fields of barley, corn, beets, vegetables, and many family gardens. The bark of young fruit trees and grape vines was frequently gnawed and consumed, killing terminals.
Depredations by this grasshopper have a long history beginning with the settling of California by the Spanish. Records as early as 1722 indicate destructive populations. In 1855 immense flights and severe damage occurred in California, Oregon, and Washington. The most recent general outbreak took place from 1955 to 1961 with annual infestations ranging from 580,000 to 4,523,000 acres. Irruptions since then have occurred on less than 500,000 acres each year.
Live weights of 20-day-old adult males reared from late nymphal instars averaged 280 mg and of females 341 mg (dry weights 103 mg and 125 mg, respectively). The nymphs were collected from a population inhabiting Jasper Ridge, Palo Alto, California on 5 July 1993 and reared on dandelion.
Food Habits
The devastating grasshopper, a polyphagous insect, consumes a variety of grasses, forbs, shrubs, and trees. Under favorable spring conditions in their natural rangeland habitat, the nymphs feed preferentially upon various legumes, filaree, and brome and barley grasses. When these plants mature and become dry, late nymphs and adults survive on green but less palatable plants such as needlegrass, Stipa spp., tarweeds, Hemizonia spp. and wild lettuce, Lactuca spp. Favorite items of food at these times are grass seeds shed naturally or dropped by harvester ants (Messor andrei) on their mounds. The late nymphs and adults have been observed feeding on the epidermis of stems of the less palatable plants and on the edges of leaves. They may reject other drought-resistant plants that commonly grow in their habitat, such as turkey mullein. They also feed on stubble and ground litter. Late nymphs assume various orientations in feeding. Two common orientations are a nearly vertical head down position as they feed on epidermis of stems and a horizontal position on the ground as they feed on litter.
After rains start in fall and growth of preferred host plants begins anew, the adults mature and reproduce. Many observations have been made of the damage caused by the migrating swarms. Migrants have been observed to feed on the leaves of grape, citrus, apple, pear, cherry, peach, apricot, prune, plum, almond, avocado, and also on cabbage, tomato, beet, beans, marigold, alfalfa, clover, timothy, corn, and barley. The list is undoubtedly incomplete and could be lengthened considerably from experiences of California growers, entomologists, and horticulturists.
Identification
The devastating grasshopper is a medium-sized grasshopper with long wings that extend beyond the end of the abdomen (Fig. 7 and 8). Along with the migratory grasshopper, it is a member of the mexicanus group of the genus Melanoplus. The males have two diagnostic characters that distinguish this species from the migratory grasshopper, which it resembles. The cerci are elongated and slender; the furculae are long, extending halfway on the supraanal plate (Fig. 9).
Both males and females of the devastating grasshopper share several characteristics. The body color is pale gray and tan with fuscous maculations; the venter of the abdomen is pale greenish yellow. The tegmen is marked by a row of conspicuous black spots (Fig. 7). The color pattern of the medial area of the hind femur consists of pale or reddish tan and fuscous patches. The hind tibiae are usually blue but may be red. In the Jasper Ridge, California site, 3 percent of males and 15 percent of females have red hind tibiae.
The nymphs are identifiable by their structure and color patterns (Fig. 1-6).
1. Head with face nearly vertical; antennae filiform, compound eyes brown with irregular ivory spots and occasional dark spots; a broad, ivory, or pale tan crescent begins on gena below compound eye and extends onto side of pronotum.
2. Hind femur with narrow dark stripe in center of medial area in instars I and II; in instars III to VI dark stripe cut in middle by wide (approximately two chevrons wide), diagonal, pale tan bar; a large pale tan patch interrupts the dark stripe at base of medial area.
3. General body color pale yellow, pale tan, or pale green.
Although the nymphs of the devastating grasshopper closely resemble those of the migratory grasshopper, they can be separated by differences in several characters that can be seen with either the naked eye or a 10x magnifier. Instar I of the devastating grasshopper is pale, has the ivory crescent clearly evident, and the medial area of the hind femur is pale yellow with a narrow dark stripe running down the middle. Instar I of the migratory grasshopper is dark with many fuscous spots, the crescent is faint, and the medial area of the hind femur is marked heavily with fuscous spots that partially conceal the dark stripe.
Two characters are useful in separating instars II-VI. The compound eyes of devastating grasshopper nymphs lack a diagonal dark bar, which is present in compound eyes of the nymphs of the migratory grasshopper. The medial area of the hind femur of the devastating grasshopper has the dark stripe interrupted by large ivory patches, while that of the migratory grasshopper has smaller light patches. (See illustrations of nymphs of the two species.)
Recent genetic studies of the two species suggest that M. sanguinipes and M. devastator are closely related and may be the same species or at least subspecies. However, because of clear structural, life history, and geographic differences, taxonomists continue to regard the two as related but separate species. Despite the controversy over species definition, the ecology and bionomics of the migratory and the devastating grasshoppers differ substantially. In California, the migratory grasshopper breeds and becomes a minor problem in irrigated alfalfa and permanent pastures (improved and irrigated), while the devastating grasshopper breeds in foothill rangelands and becomes a major problem when bands and swarms migrate into cropland.
Dispersal and Migration
When populations irrupt and rangeland forage becomes depleted, the devastating grasshopper becomes a highly migratory insect. Bands of older nymphs crawl and hop while swarms of adults fly to the valleys. Nymphs usually migrate downhill toward more succulent green vegetation. They follow ravines and drainages toward cultivated crops, often migrating 5 miles or more during this stage. Extensive movements of nymphs took place in Alameda and Butte counties in California on 13 June 1957 when grass had dried on the hillsides but swales were still green.
Adult migrations are less predictable and may be delayed. Between 25 July and 8 August 1957, a spectacular migration of adults occurred from the California Range Experiment Station at Hopland. Adults are strong fliers and swarms migrate 15 or more miles in a single day. In this stage they have been observed to travel a distance of at least 30 miles. Attractive landing sites are fields of yellow stubble of barley, oats, and wheat. From these fields the devastating grasshopper disperses to fruit and vegetable crops. Even though the major pest status of this grasshopper results from its migrations, no special study of this behavior has been made. Some entomologists speculate that every year a part of each population migrates, but the migrants usually go unnoticed because of small numbers.
When flushed, nonmigratory adults travel 3 to 6 feet at heights of 4 to 12 inches. Their flight is straight and silent; however, flight noise or crepitation was heard once in San Mateo County, California.
Hatching
The hatching period, which ranges from 50 to 103 days, starts in late April and may extend to late July. This is a prolonged hatching period compared to that of several other species (e.g., Camnula pellucida and Oedaleonotus enigma) that occur in assemblages of California rangeland grasshoppers. The difference is probably due in part to the lateness of oviposition and greater depth of the eggs in the soil of the devastating grasshopper. The total heat units required for development and hatching of the eggs of all three species are probably similar.
Nymphal Development
The nymphs begin development when forage is green and succulent but a majority are often in the nymphal stage (instars IV, V, and VI) when the forage has matured and dried. Nutritional stress evidently prolongs the nymphal period as some nymphs may still be found in mid October. Both males and females require six instars to complete their nymphal development. The first nymphs that appear during the last week of April may become adult by July 1. This means that precocious adults take about 70 days to reach the fledgling stage.
Adults and Reproduction
Among rangeland species, the devastating grasshopper has a unique life history. Females enter a reproductive diapause during the summer months and must survive on less palatable forage to tide them over to the reproductive period, which begins in late September. Laboratory experiments have shown that development and maturation of eggs within the females are triggered by decreasing day length. Usually fall rains arrive at this time, stimulating sprouting and growth of preferred food plants. The usual coincidence of the breaking of reproductive diapause and the availability of nutritious host plants allows the females to oviposit prolifically. Dissection of females collected at the Hopland Range Experiment Station in Mendocino County, California and at Mission Peak study area in Alameda County, California, revealed that mature eggs were present in the ovaries at the end of September and in October, 1963.
The females oviposit in restricted locations within their normal rangeland habitat. They prefer well-drained hillocks, ridges, slopes, and banks of ravines where soil is gravelly. Many pods are deposited within relatively small areas (egg beds). Only a few pods are deposited in surrounding soil. Pods are deposited close to the basal growth of forbs such as tarweeds and Russian thistle. Some are laid among the roots of filaree or in small, well-drained bare spots. Pods are 3/4 to 7/8 inch long, slightly curved, and contain 20-31 eggs (Fig. 10). The eggs are pale yellow and 3.9-4.4 mm long.
No study of courtship of the sexes has been made nor is it known whether the males, like the females, have a reproductive diapause.
Population Ecology
In their natural rangeland habitats in the foothills of the coastal states, populations of the devastating grasshopper persist over many years. Astonishingly large changes in densities occur with time. Grasshoppers may increase from noneconomic numbers to as many as 100 per square yard in just three years. Before the severe outbreak of 1957-58, survey entomologists began to notice increases in densities in the Sierra Nevada foothills in 1952. In 1953 and 1954 the sizes of populations were rated about the same as in 1952, but in 1955 the densities of populations increased dramatically and the economically infested acres expanded by five fold, from 220,000 acres in 1954 to 1,264,000 acres in 1955. The sudden increases in both densities and infested acreage suggest that populations had experienced especially favorable conditions for growth. Although populations grew at a slower rate during the next three years, they continued to increase. Two other species, Oedaleonotus enigma and Camnula pellucida, contributed to the outbreak, but the devastating grasshopper was the primary pest. In 1959 a fungal disease (Entomophaga grylli) killed many grasshoppers, reducing some populations by as much as 90 percent. The disease and a subsequent drought led to a gradual decline of the outbreak during the next six years.
Like other rangeland grasshopper assemblages, those in the foothills of California consist of several species. The devastating grasshopper often dominates and coexists with O. enigma, C. pellucida, Dissosteira pictipennis, and several other species. In habitats less favorable for the devastating grasshopper, other species may dominate. In montane grasslands C. pellucida usually dominates, while in valley and low foothill habitats O. enigma often dominates. Migrants of the devastating grasshopper that invade crops do not successfully colonize these valley habitats, but the reason for this is unknown.
Daily Activity
Behavior of the late nymphs and young adults was observed on Jasper Ridge in a serpentine grassland habitat from the 3 to 5 July 1993. The ridge is a low-lying foothill (maximum elevation 620 feet) on the eastern side of the Santa Cruz mountains with a Mediterranean-type climate. Skies are generally clear and virtually no rains fall during the nymphal development of the devastating grasshopper. Sampling of the resident population of devastating grasshoppers indicated a density of eight per square yard (late nymphs and young adults).
In early July, ground temperatures during the night fall to 60°F and air temperatures to 50°F. Approximately one hour after sunrise the grasshoppers begin to move from overnight retreats and crawl to sunny spots to bask. Some crawl up stems of dead weeds or culms of grasses, while others stay on the ground. They turn a side perpendicular to the rays of the sun and lower the associated hindleg to expose the abdomen fully to the warming rays. They bask for a period of two hours from 7 to 9 a.m. DST. However, feeding may start as early as 8 a.m., and feeding of different individuals may be observed for two or more hours.
The feeding period ends when rapidly increasing temperatures of the habitat force them to take evasive actions. By mid morning, surface temperatures of the black, gravelly serpentine soils rise to over 130°F and later exceed 160°F. To avoid high ground temperatures the grasshoppers climb forb stems and grass culms and rest vertically head-up in the shade at heights of 2 to 9 inches. When temperatures moderate in late afternoon (5 p.m. DST), the grasshoppers feed again. This second period of feeding lasts for approximately two hours. The grasshoppers have no second daily period of basking. After feeding they seek shelter under canopies of vegetation and may back into small depressions. They begin this movement as early as 7 p.m. when ground surface temperatures are in the 80s and air temperatures are 67° to 71°F.
References
Cooperative Economic Insect Report 1952-1975. USDA APHIS PPQ. Vol. 2-25.
Cooperative Plant Pest Report 1976-1980. USDA APHIS PPQ. Vol. 1-5.
Middlekauff, W. W. 1964. Effects of photoperiod upon oögenesis in Melanoplus devastator Scudder (Orthoptera: Acrididae). J. Kansas Entomol. Soc. 37: 163-168.
Orr, M. R., A. H. Porter, T. A. Mousseau, and H. Dingle. 1994. Molecular and morphological evidence for hybridization between two ecologically distinct grasshoppers (Melanoplus sanguinipes and M. devastator) in California. Heredity 72: 42-54.
Rentz, D. C. F. and D. B. Weissman. 1981. Faunal affinities, systematics, and bionomics of the Orthoptera of the California Channel Islands. Univ. California Publ. 94: 1-240.
Weissman, D. B. and D. C. F. Rentz. The Orthoptera of Stanford University's Jasper Ridge and neighboring Palo Alto, California. Wasmann J. Biol. 35: 87-114.
Wilson, C. C. 1947. Control of the devastating grasshopper in California. Bull. California State Dept. Agric. 36(3): 97-102.
Next Species in Subfamily: Melanoplus differentialis

