UW Researchers Resolve Controversy Over Energy Gap of a Van der Waals Material
Published January 15, 2021
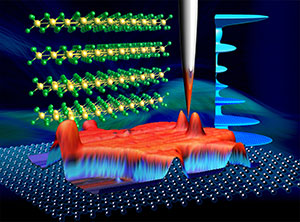
Previously controversial values of the energy gap of a van der Waals material -- chromium tribromide -- were reported based on various optical measurements. A University of Wyoming faculty member and his research team used scanning tunneling microscopy and spectroscopy measurements that clearly reveal a much smaller energy gap value and resolved the controversy.
“Our results settled a long controversy on one important material property -- the energy gap of the material,” says TeYu Chien, an associate professor in the UW Department of Physics and Astronomy. “Our scanning tunneling microscopy and spectroscopy measurements clearly revealed that the energy gap is around 0.3 electron volt (eV), which is much smaller than those measured by optical methods, which ranged from 1.68 to 2.1 eV.”
Chien says his team’s data further explain those previous optical measurements as being the transitions from various conduction and valence band features rather than detecting the energy gap of the material.
Van der Waals materials are made up of strongly bonded two-dimensional layers that are bound in the third dimension through weaker van der Waals forces. For example, graphite is a van der Waals material that is broadly used in industry in electrodes, lubricants, fibers, heat exchangers and batteries. The nature of the van der Waals forces between layers allows researchers to use Scotch tape to peel the layers into atomic thickness.
Chien is the corresponding author of a paper, titled “Small Energy Gap Revealed in CrBr3 by Scanning Tunneling Spectroscopy,” that was published Dec. 8 in Physical Chemistry Chemical Physics, an international journal for the publication of cutting-edge original work in physical chemistry, chemical physics and biophysical chemistry. The paper has been selected into the journal’s “hot articles,” a theme collection featuring the hottest work published in Physical Chemistry Chemical Physics. This work also will be featured on the outside front cover of the upcoming print edition.
Dinesh Baral, a UW graduate student from Nepal, was the lead author of the paper. He carried out the experimental works on scanning tunneling microscopy and spectroscopy measurement, and data analysis. Other researchers who contributed to the paper are Assistant Professor Jifa Tian, Professor Yuri Dahnovsky and Jinke Tang, a professor and department chair, all from UW’s Department of Physics and Astronomy.
Graduate students involved with the research included Zhuangen Fu and Aaron Wang, both from China; Uppalaiah Erugu, of India; Rabindra Dulal and Narendra Shrestha, both of Nepal; and Andrei Zadorozhnyi, of Russia.
Since the first isolated graphene -- atomically thin graphite -- in 2004, various van der Waals materials with properties of metal, semimetal, semiconductor, insulator and superconductor have been confirmed. The magnetic van der Waals materials did not join the graphene family until 2017.
Chromium trihalides are one family of the key van der Waals magnetic materials and have been used to explore the potential for spintronic applications, in which the magnetic moment of the electron is used for computing and information storage rather than using the charge properties of the electrons for conventional electronics.
Because van der Waals materials have very weak interlayer interactions and relatively stronger intralayer atom-to-atom bonding, this allows researchers to peel them and stack them for any combination of materials at atomic thickness.
“This peeling of the van der Waals materials is like peeling the onion skins, but at the atomic level,” Baral explains.
Scanning tunneling microscopy and spectroscopy is an imaging tool capable of measuring atomic resolution images, along with the electronic properties in that scale. Chromium tribromide flakes were peeled off from bulk crystal into atomically thin thickness and transferred onto a conducting substrate, such as highly oriented pyrolytic graphite, for the study.
“The understanding of the energy gap of chromium tribromide resolves the existing controversy for the scientific community,” Chien says. “This also is the key in better controlling the spintronics devices involving chromium tribromide.”
The results of the study will provide researchers a better understanding of this important material for applications in spintronics and quantum materials, Chien says.
“Materials having such properties have potential applications in engineering at minimizing the size of the electronic and spintronic devices toward atomic level,” he says.
The project was funded by the U.S. Department of Energy, Office of Basic Energy Sciences, the Division of Materials Sciences and Engineering, and EPSCoR (Established Program to Stimulate Competitive Research). Baral was supported by an Energy Graduate Assistant Fellowship from UW’s Office of Academic Affairs.

