Leonard Research Group
Research
Our research focuses on solid state materials synthesis. We currently have several projects dealing with the synthesis of metal boride, carbide, and nitride nanomaterials through new low temperature methods.
Facilities available at the University of Wyoming
Synthetic control over morphology and crystal structure.
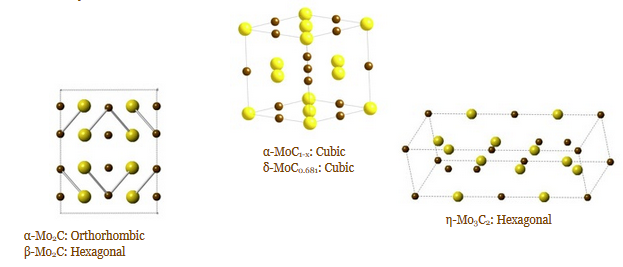
Many applications depend on the crystal structure of the material and the crystal surfaces or faces present. To date, most of the metal carbide synthesis has focused on thermodynamically stable phases due to the extremely high temperatures required for their synthesis. In addition, high temperature synthesis leads to particle agglomeration and severely limits control over particle size and morphology. We are currently developing new low temperature methods to synthesize these compounds with control over not only particle size but also crystal structure. By taking advantage of fluxes, amine reductions, and high temperature solution synthesis, we have been able to synthesize a number of metal carbides at greatly reduced temperature compared to traditional synthesis.
Catalysis and support materials
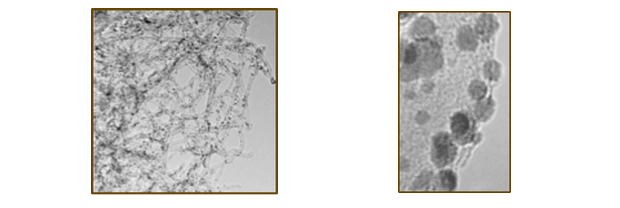
We are investigating carbide and nitride materials as electrocatalysts and catalyst supports for fuel cell and electrolyzer applications with the goal of developing new materials that are not only cheaper and more robust than current catalysts, but also more catalytically active. These materials will reduce cost by eliminating some or all of the precious metals, all while improving the efficiency, stability, and resistance to poisoning. Carbides are well known to be conducting and chemically stable in acidic environments that are present in fuel cells and have been reported to exhibit Pt like chemical reactivates and electronic properties making them ideal candidates for elctrocatalyst materials. We are synthesizing bimetallic carbides using a variety of techniques, and are interested in mechanistic studies of these reactions which will allow the development of a general synthesis scheme with precise composition and size control. This will give us access to hundreds of different multi-metallic carbides nanoparticles which can be then tested as catalysts.
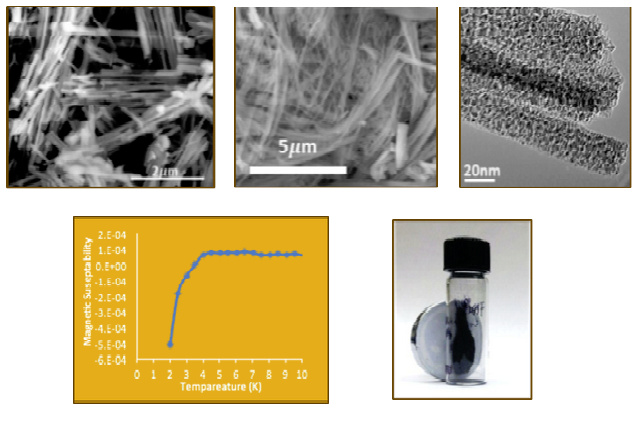
Several metal carbide compounds possess interesting physical properties. These compounds are known for their extreme hardness, high melting points, chemical inertness and strength. Tantalum carbide and hafnium carbide have the highest melting points of any binary compounds at 3,983 °C (7,201 °F) and 3,928 °C (7,102 °F), respectively. Additionally, tungsten carbide is one of the hardest known materials and is nearly as hard as diamond. What is often less studied and mentioned is their magnetic and electronic properties. Most carbides are good electrical and thermal conductors and a few are known superconductors. δ-MoC0.681 is the highest known superconducting carbide compound and is very sensitive to crystal structure and carbon content. Finally, late transition metal and rare earth carbides are being investigated as high performance permanent magnets due to their high Curie temperatures and saturation magnetization. In addition to synthesizing these materials, we are also studying their size/shape dependent physical properties.