Contact Us




Department of Molecular Biology
University of Wyoming
Department #3944
1000 E. University Ave.
Laramie, WY 82071
Phone: (307) 766-3300
Fax: (307) 766-5098
Email: mbiology@uwyo.edu
How do protein kinases direct the busy transportation highways inside cells?
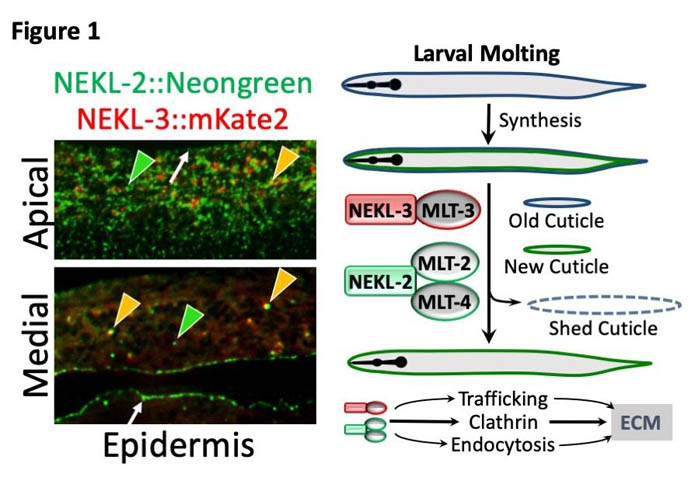
One of the surprising findings to come out of our mutant screens for molting-defective worms was the identification of several protein kinases, NEKL-2 and NEKL-3. Protein kinases regulate the activities of many different types of proteins in cells, leading to a wide range of outcomes.They do this by attaching phosphates to certain amino acid side chains on their target proteins. In addition, the same screens found three binding partners of the NEKLs, MLT-2, MLT-3, and MLT-4 (Figure 1).The MLTs all contain ankyrin repeat domains, which are commonly found on proteins that function as molecular scaffolds. Scaffolding proteins coordinate protein interactions and control the subcellular localization of other proteins. The NEKLs and MLTs are well conserved, meaning that they have corresponding close relatives in mammals, termed homologs or orthologs. These homologs are structurally similar and likely to carry out very similar if not identical cellular functions. The reason our finding was surprising is that based on the scientific literature describing the functions of mammalian NEKLs and MLTs, it wasn’t clear how or why these proteins would be involved in worm molting. As sometimes happens, however, we found that the NEKLs and MLTs have a cellular function that had been largely overlooked by the mammalian studies but is almost definitely conserved. Our findings highlight (1) why non-biased approaches, such as genetics, can be a very potent means for identifying novel gene functions and, (2) why scientists taking different paths and using different organisms can often be so valuable.
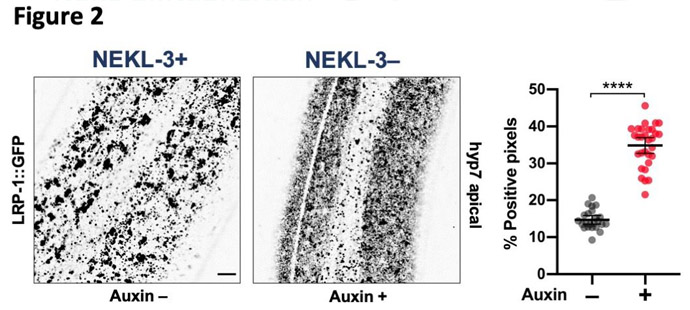
What we found is that NEKLs and MLTs act together to control intracellular trafficking. Most cells are constantly making new proteins, turning over old proteins, and moving proteins to different locations within cells as required for specific functions at specific times. For example, during molting new proteins must be synthesized, transported to the surface, and then released in order to build a new cuticle. At the same time, proteins from the surface must be internalized, including some of the proteins that made up the old cuticle. The former process, exocytosis, and the latter process, endocytosis, must also be perfectly balanced in order for cells to remain a constant size. We discovered that NEKLs and MLTs are required for a particular kind of endocytosis involving the highly conserved molecule, clathrin. Clathrin forms a basket-like structure on the surface of cells (Figure 2) and most internalized surface proteins are imported through this pathway. One of the defects we identified in worms that lack NEKL function is that disassembly of clathrin baskets, immediately following internalization, appears to be dramatically slowed compared to the state in normal worms. This ‘uncoating step’ is essential in order for internalized surface vesicles and their associated proteins to be routed deeper into the cell through the endocytic pathway.
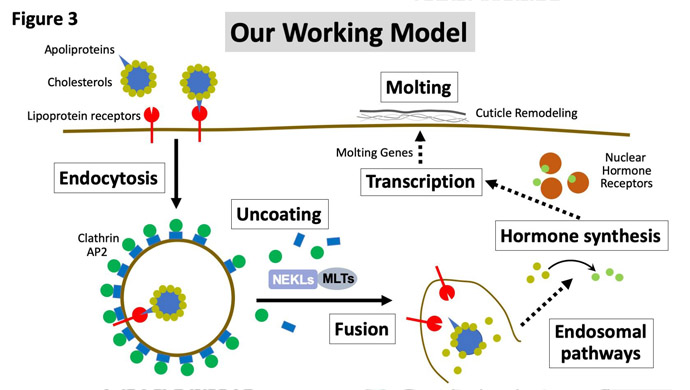
We are currently determining exactly how NEKLs and MLTs regulate endocytosis, and our findings thus far suggest that they could be acting at multiple discrete points in the endocytic pathway. Determining the specific functions and kinase targets of the NEKLs is one of our foremost goals. Our overall working model is that NEKLs are defective at molting because of a failure to internalize cholesterol from the environment (Figure 3). Cholesterol is a required precursor for making steroid hormones, which are known to be critical in the control of molting cycles. Because worms can’t synthesize their own cholesterol, they need to get it from the environment, and they appear to do this by internalizing it through their skin cells. We have found that NEKLs are needed for the internalization of cholesterol-binding proteins (Figure 3) and that loss of NEKL activity leads to a failure to turn on steroid-responsive genes needed for molting.
Another exciting result was our finding that human NEKL proteins could substitute functionally for worm NEKL proteins in living worms. We did this by removing the worm NEKL proteins and replacing them with the human versions. We found that the human proteins could restore normal molting and intracellular trafficking in worms that lacked their own NEKL proteins. We are also collaborating with other research groups to study trafficking defects that result when human NEKL proteins are depleted from human cells. Our goal is to carry out parallel studies in worms and human cell lines to characterize and correlate NEKL trafficking functions in these two complementary systems. It’s also important to note that mutations in human NEKL proteins are associated with a number of diseases including cancer and developmental defects. By studying their functions we hope to gain a better understanding of the role of NEKL proteins in biology and human disease, potentially paving the way for new diagnostic tools or treatments.
Contact Us




Department of Molecular Biology
University of Wyoming
Department #3944
1000 E. University Ave.
Laramie, WY 82071
Phone: (307) 766-3300
Fax: (307) 766-5098
Email: mbiology@uwyo.edu