FacultyStephen W. Santoro
Graduate Neuroscience Program
 |
Publications
|
|
Research
The mammalian nervous system is profoundly altered by experience – a process that is essential for development, adaptation and learning. Disruption of experience-dependent changes is thought to underlie certain neurodevelopmental disorders. Despite their importance, the mechanisms by which experiences are converted into long-term neuronal changes are not well understood. A goal of research in my laboratory is to elucidate molecular mechanisms that orchestrate experience-dependent changes in the nervous system. This goal is approached using a variety of modern molecular and genetic tools. In cases where technologies do not exist for answering questions of interest, new ones are developed. Research in my laboratory employs the experimentally tractable mouse olfactory system, which exhibits many of the types of experience-dependent developmental and adaptive changes that occur elsewhere in the nervous system. These investigations are expected to provide a basis for broader mechanistic studies within the brain. Current areas of research:
1. How are the birth and survival of olfactory sensory neurons controlled by olfactory
experience? What is the function of this type of plasticity?
Olfaction in mammals is mediated by a population of olfactory sensory neurons, each of which expresses just one of ~1000 olfactory receptor proteins that are encoded in the genome. Olfactory activity is known to induce dramatic changes in the abundance of different sensory neuron subtypes (i.e., neurons that express different olfactory receptor proteins) in an individual’s olfactory epithelium. This process is mediated by differential rates of turnover (i.e., the rates of birth and survival) among the different neuron subtypes. The mechanisms by which neuron turnover is controlled at the molecular level are poorly understood – a deficiency that is a focus of my laboratory. We hypothesize that changes within the population of olfactory sensory neurons may facilitate adaptation of an individual’s olfactory system to its unique olfactory environment. 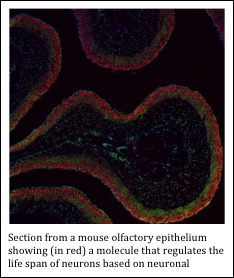
2. How does odorant receptor activity control axon guidance and refinement?
The axons of all olfactory sensory neurons that express a particular odorant receptor subtype project to one of two stereotyped positions within the olfactory bulb. Surprisingly, the odorant receptor plays an essential role in guiding a neuron’s axon to the appropriate position. How does this work? It appears that each odorant receptor protein type has a different level of constitutive (odor-independent) activity, which is required for the proper guidance axonal projections from the olfactory epithelium to the olfactory bulb. Upon reaching the olfactory bulb, refinement of axonal projections into a single glomerular structure requires receptor activity that appears to be odorant-dependent. Olfactory axon guidance and refinement are known to involve the activity-dependent expression of effector molecules, but little is known about how the expression of these molecules is regulated at the levels of transcription and translation. Previous studies have made the surprising observation that odorant receptors localize to axon termini (both mRNA and protein), hinting at a possible role for this localization in activity-dependent axon guidance and/or refinement. These findings are especially intriguing in light of recent discoveries that local translation within axons plays a central role in axon guidance. Current projects in the lab are designed to elucidate mechanisms by which receptor activity orchestrates axon guidance and refinement. These projects explore the hypotheses that these processes are controlled, at least in part, at the levels of cellular transcription and local protein translation within sensory neuron axons.
3. What are the identities and functions of odorant receptors that detect gender-specific odors?
In the course of investigating experience-dependent changes in the olfactory system, we identified a large number of genes that are expressed differently in the olfactory epithelium of male and female mice that are housed under specific conditions. These include a number of genes that encode odorant receptors. Preliminary findings indicate that these receptors detect odors that are specific to males or females. The physiological and behavioral functions of these receptors (and other genes identified) are areas of interest in the laboratory.
4. New technologies to enable cell-level mapping of gene expression in complex tissues
within the nervous system.
The complexity of the mammalian nervous system is unparalleled in nature. Technologies that enable the resolution of this complexity are extremely valuable for understanding how neurons and circuits change with experience. A goal of my laboratory is to develop technologies that will enhance our ability to study how gene expression is regulated based on experience, at both the transcriptional and translational levels, and how these processes go awry in neurodevelopmental disorders. Education and training:
Undergraduate: University of Wyoming; Majors: Chemistry, Molecular Biology; Research area: Asymmetric organic catalysis (advisor: Robert Corcoran) Graduate: The Scripps Research Institute (advisor: Gerald Joyce); Research area: Directed evolution of nucleic acid-based enzymes Postdoctoral - chemistry: The Scripps Research Institute (Advisor: Peter Schultz); Research area: Directed evolution of protein-based enzymes Postdoctoral – molecular neurobiology: Harvard University (Advisor: Catherine Dulac); Research area: Experience-dependent changes in the mammalian olfactory system Laboratory Personnel:
Students interested in undergraduate, graduate or postdoctoral research opportunities should contact Dr. Santoro directly at ssantoro@uwyo.edu. |
|
