FacultyFrancis W. (Bill) Flynn
Graduate Neuroscience Program
 |
PublicationsPost-docs
|
Research Projects

Neurotransmitter control of vasopressin release:
In recent years we have become interested in the role of a specific receptor- the neurokinin 3 receptor (NK3R), in the control of vasopressin release. We had previously established that this receptor plays a critical role in the ingestion of salt solutions and that the activation of the receptor affected the central processing of salt taste. The neurohormone vasopressin complements behavioral responses (ingestion of salt) to maintain body sodium levels by affecting the retention of water.
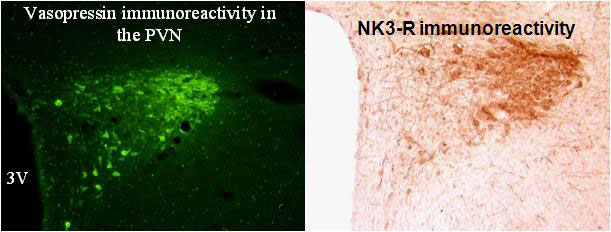
Immunohistochemistry showed that the majority of vasopressin neurons in the paraventricular nucleus express the NK3R. Furthermore, injections of agonists for the NK3R stimulate vasopressin release and blockade of the receptor prevented the release of vasopressin to hyperosmolarity, hypotension and cholecystokinin injection.
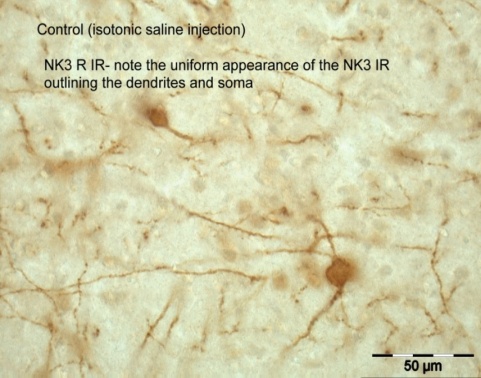
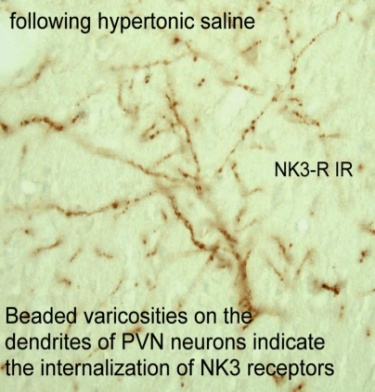
The NK3R is a G protein coupled receptor and after a ligand binds the receptor, the receptor is internalized to the cytoplasm. Receptor internalization is a pharmacologically specific marker of receptor activation. We used this technique to show that hyperosmolarity activates NK3R expressed on neurons in the paraventricular nucleus. Internalized receptors cause dendrites to take on a bead like appearance, as shown in the figure. Neurokinin B (NKB) is the endogenous transmitter that binds the NK3R and projects now focus on the NKB afferent pathways to the hypothalamus and specifically vasopressin neurons.
Nuclear trafficking of a membrane NK3 receptor:
Immunohistochemistry revealed that following an osmotic challenge, the tachykinin NK3 receptor that is expressed on vasopressin neurons is activated, internalized to the cytoplasm and then transported to the cell nucleus!In order to follow the trafficking of the NK3R we transfected cells with NK3R tagged with the green fluorescent protein (GFP). The movie shows that NK3R-GFP was membrane and cytoplasmic initially. But after the administration of a NK3R agonist, the GFP-NK3R appears in the nucleus of the neuron.
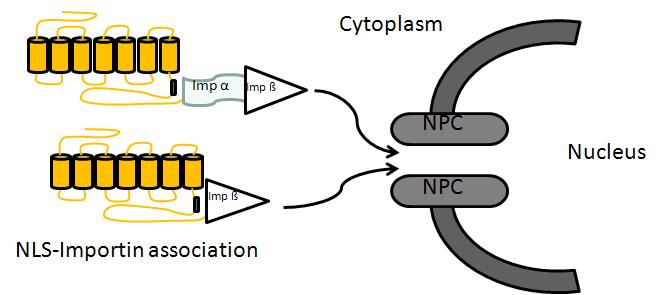
Experiments are aimed at identifying how this receptor protein gets into the nucleus. This receptor contains the peptide sequence, the nuclear localization sequence, needed for transport to the nucleus. With Dr. Dane Jensen we used co-immunoprecipitation to show that translocation of the NK3R into the nucleus involves the importin pathway. Other steps in how this protein moves through the cytoplasm and ultimately into the nucleus remain under investigation.
What is a g-protein coupled receptor is doing in the cell nucleus?
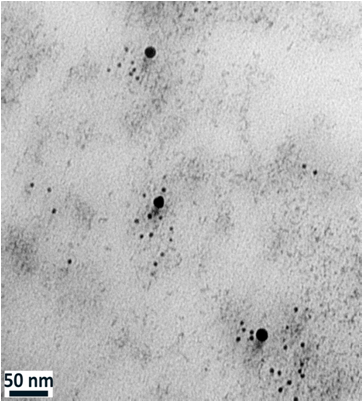
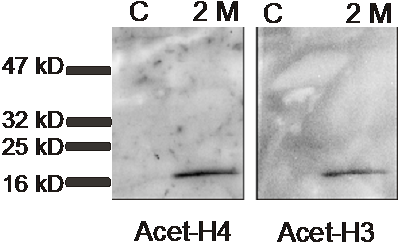
We use immuno-electron microscopy to study the distribution of the NK3R in the nucleus. The adjacent electron image from the nucleus of a neuron shows two different sized gold beads. The larger beads are attached to antibodies that detect the NK3R and the smaller beads are attached to antibodies that recognize acetylated histones. Histones are the backbone of DNA and acetylation of histones is associated with active gene transcription. Co immunoprecipitation showed that in rats treated with 2 M salt solution, but not in control (C) rats, NK3R associated with acetylated histone H3 and H4. The band in the western blot image is detecting the histone that was “pulled down” with NK3R. A major question is what specific genes are being affected by NK3R binding to histones. We know that hyperosmolarity up-regulates many genes, including that for vasopressin, and we hope to identify which of those genes are being controlled directly by NK3R.