

Lon Kendall
Lon Kendall, DVM, PhD, DACLAM - received his veterinary degree from Colorado State University in 1994, completed a residency in laboratory animal medicine in 1997, and completed a PhD in veterinary pathobiology in 2000. He has over 25 years of experience in laboratory animal medicine and program management. He has served as the AV for several institutions including UC Davis, University of Pacific, University of Northern Colorado, and a few contract research organizations. He is currently the director of Laboratory Animal Resources and oversees the animal care program at Colorado State University, which includes a diverse population of species and research including those in biocontainment. His expertise is in analgesia of rodents, infectious diseases in rodent models, animal welfare and regulatory compliance.
1. IACUC Submission Due Dates Have Changed
To improve pre-review processes and ensure compliance with federal regulations, the deadline for submitting new and de novo protocols has been moved to the last Tuesday of each month (previously the first Tuesday). This change provides the Attending Veterinarian and IACUC office with sufficient time to conduct thorough pre-reviews as required by federal regulations. This adjustment will help streamline the approval process and reduce delays in research initiation. Please refer to the updated IACUC calendar in few sections below for specific submission dates and contact the IACUC office with any questions.
2. Registration for DEA-Controlled Substances
Researchers working with DEA-controlled substances are required to register according
to both DEA and Wyoming Researchers using DEA-controlled substances must complete
both DEA and Wyoming state registration requirements. Please follow all necessary
steps to ensure compliance. Registration instructions are provided below on this webpage
under the “Medication and DEA Controlled Substances” section.
3. Steam Shutdown - Thursday, June 26th - Wednesday, August 6th.
General notes about the annual steam shutdown:
Please send an email to iacuc@uwyo.edyu if you have any questions.
For information on this mandatory program for animal users please visit here.
All animals used for research, breeding, or teaching at UW must be included in an IACUC protocol that has been reviewed and approved before any activities begin. Principal Investigators (PIs) should submit their IACUC protocols through ROAMWyo well in advance, allowing sufficient time for the IACUC to complete its review and approval process.
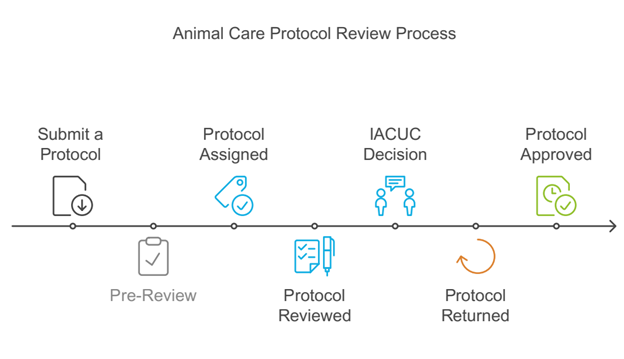
1. Submit a Protocol
PIs must submit the protocol (new protocols, continuing reviews, de novo, or amendments) to the UW IACUC via ROAMWyo. Explanatory videos and SOPs are available (RoamWyo – link below the page). Researchers should ensure that all required personnel, animal training, animal occupational health clearances, and permits are included and up to date.
2. Pre-Review
For protocols involving USDA pain categories D and E, the Attending Veterinarian will also conduct a pre-review, and it is recommended that these protocols be submitted 1-2 weeks before the deadline. The PI will be contacted with any suggested or required changes prior to the IACUC review.
3. Protocol Assigned
Depending on the protocol category (amendments, continuing review, new protocols, or de novo, the protocol will be assigned for either DMR or FCR at the next convened meeting.
• FCR (Full Committee Review): Occurs at the monthly IACUC meeting, deadlines for submission and the meeting schedule listed below.
If a protocol is submitted after the deadline, it will not be reviewed until next month’s meeting. For further questions, contact iacuc@uwyo.edu.
• DMR (Designated Member Review): Occurs at any time during the month and does not wait until the IACUC meeting.
Amendments and Continuing Reviews typically go through DMR and can be submitted at any time during the month. However, the IACUC makes the final determination as to whether DMR is appropriate.
4. Protocol Reviewed and IACUC Decision
The protocol is carefully reviewed to ensure compliance with animal welfare regulations and The Guide. The protocol may be approved, require modifications (to secure approval), disapproved, or tabled (defer until future meeting).
5. Protocol Returned
Reviewer concerns are communicated to the PI via ROAMWyo. The PI or Co-Investigator is responsible for responding to the committee’s questions and revising the protocol through ROAMWyo. The average turnaround time for de novo reviews and new protocols is approximately 40 days, though it may take longer depending on the complexity of the protocol.
6. Protocol Approved
If there are no outstanding issues and the protocol meets IACUC requirements, the application is approved.
NIH uses Just-In-Time (JIT) procedures for most programs and award mechanisms. Just-In-Time procedures allow PI to defer verification of IACUC approval for the project's proposed use of live vertebrate animals until after completion of the peer review and just prior to funding. Applicants with a potentially fundable score will receive a JIT request from an NIH system generated e-mail or directly from an agency contact via e-mail or telephone. When a JIT request is received immediate submission of the animal protocol is advised, along with an email to iacuc@uwyo.edu. Protocols may be submitted and reviewed by the IACUC prior to receipt of the JIT request.
IACUC approved animal protocols must undergo complete review at least once every three years (PHS Policy, IV.C.5.). To avoid any interruption in animal work, the de novo review should be submitted as soon as the PI receives the ROAMWyo notification of the expiration timeline (usually 90 days before the protocol expires).
| Type of Review | Average Days to Approve |
| Amendments | 23 days |
| Continuing Review | 27 days |
| New | 52 days* |
| De Novo | 34 days* |
*For New and De Novo, these metrics are applied to protocols submitted on time for the IACUC monthly meetings – the schedule is described below.
Time period: July to October 2024, measured in calendar days (average). Amendments exclude administrative amendments.
Faster reviews:
· Principal investigator (PI) is quick to respond to email inquiries.
· There are no or few questions from reviewers.
· Submission is a minor amendment. · Related reviews are obtained first, if required (e.g., those from RSC and IBC)
· Project is non-invasive or not complex.
· Submission is not called to full committee review.
Slower reviews:
· PI is slow to respond to email inquiries.
· Reviewers have many questions.
· Submission is extensive and could potentially involve pain or distress.
· Protocol depends on IBC, RSC or other outside reviews that have not been completed.
· Submission requires full committee review.
Amendments and Continuing Reviews are accepted on a rolling basis. New protocols and de novo reviews that require full committee review and are submitted before the deadlines listed below will be considered at the next regularly scheduled meeting.
Support & Reporting Issues
· For Animal Oversight questions, contact IACUC@uwyo.edu. For ROAMWyo inquiries, email roamwyo@uwyo.edu.
· Report minor issues (typos, grammar, etc.) via this Qualtrics survey to help us improve the module. - report it here
· For major issues (logic errors, submission problems, etc.), email IACUC@uwyo.edu directly.
Drugs that need a prescription can be requested through the UW Attending Veterinarian (AV) via email at lkendal2@uwyo.edu. Only drugs listed in your approved IACUC protocol may be ordered. PIs should be able to order non-controlled substances from pharmaceutical vendors such as MWI or Covetrus.
For Non-Controlled Substances:
Any drug requiring a prescription must first be requested through the UW Attending
Veterinarian (AV) by emailing lkendal2@uwyo.edu. Only drugs explicitly listed in an
approved IACUC protocol are eligible for ordering.
For Controlled Substances:
PIs must be properly registered and licensed to handle controlled substances, which
includes obtaining the necessary state and federal (DEA) registrations. Once properly
licensed, PIs may place orders for controlled substances listed on their license through
approved pharmaceutical vendors. Currently, PIs may only order controlled substances
through Midwest Veterinary Supply. For directions and required forms, please contact
iacuc@uwyo.edu directly.
UW requires that each PI obtain their own registration and licensure, as controlled substances obtained under one individual's DEA registration typically cannot be transferred to another PI who is not DEA registered. Licensed researchers (PIs) are ultimately responsible for the management of controlled substances acquired under their DEA registration or license.
For more information regarding controlled substances, including licensing, use, storage,
and disposal requirements, please see the section below.
Need Help?
For any questions about medication ordering procedures - including licensing, tracking,
and use of controlled substances - please contact iacuc@uwyo.edu. For questions regarding
disposal, please contact the UW Safety Office - uwehs@uwyo.edu.
Overview
Controlled Substances are compounds subject to the jurisdictional control of the Drug Enforcement Agency (DEA) under Title 21, Chapter II, Parts 1300-end of the Code of Federal Regulations. The DEA classifies drugs into five schedules based on their medical use and potential for abuse. Schedule I drugs have no accepted medical use and a high potential for abuse (e.g., heroin), while Schedule II drugs have medical uses but a high abuse potential (e.g., oxycodone). Schedule III drugs have moderate abuse potential (e.g., anabolic steroids), Schedule IV have a low abuse potential (e.g., diazepam), and Schedule V have the lowest potential for abuse (e.g., cough preparations with limited codeine). DEA registration is required for handling these substances in research or clinical settings.
| DEA Controlled Substances | Non-DEA Controlled Substances |
|
Schedule II: • sodium pentobarbital • fentanyl • morphine Schedule III: • ketamine • buprenorphine • tiletamine + zolazepam • thiotetrabarbital • thiopental Schedule IV: • butorphanol • diazepam • midazolam • lorazepam • chloral hydrate • methohexital • Phenobarbital |
Anesthetics: • isoflurane •propofol Analgesics: • meloxicam • carprofen Antibiotics: • enrofloxacin • cefazolin Other Medications: • acepromazine • atipamezole |
- An alphabetical list of all controlled substances can be found in the DEA orange book 2024
- Drug Enforcement Administration Diversion Control Program - This website provides information including listings of all controlled substances in alphabetical order, links to 21CFR1300-end, plus online informational documents, brochures and forms.
- For more information about the regulations, please see Code of Federal Regulations (CFR) Title 21 §1307.22. and the DEA Researcher’s Manual.
1. Current IACUC Approval
All research and teaching activities involving controlled substances must have current and active IACUC protocol approval covering the usage of the requested controlled substances. Once approved, the DEA registrant must purchase controlled substances from licensed vendors using their own registration number. The registrant cannot purchase controlled substances for others or for unapproved activities.
2. Licensing and Registration for Researchers Working with Controlled Substances
Step 2.1. Wyoming Controlled Substances Registration (CSR)
Prior to applying for DEA registration, PIs must first obtain a Wyoming Controlled Substances Registration (CSR) from the Wyoming State Board of Pharmacy pursuant to Wyo. Stat. § 35-7-1024 and the Board's Rules and Regulations. Each lab would need to have its own registration under the PI responsibilities. Applications are available on the Board's website at https://pharmacyboard.wyo.gov/licensing/controlled-substance-reg.
Step 2.2. Federal DEA Registration
Each researcher who intends to use DEA controlled substances in their laboratory must obtain and maintain their own registration with the DEA. The registration allows the researcher to purchase, use, and dispose of controlled substances. The registration and licenses must be accessible upon request for inspection. Registered researchers must comply with all state and federal regulatory requirements while working with controlled substances.
Information on licenses and the application can be found on the DEA website and in the document - Obtaining a DEA-Controlled Substance License.
3. Storage and Security of Controlled Substances
All controlled substances must be securely stored with access limited to authorized individuals per DEA regulations. Key storage and security requirements include:
· Must be stored behind a minimum of two (2) locks (i.e. locked safe in a locked room).
· Both the room and safe must stay locked except when being used.
· The safe must be secured to the floor or building structure.
· Key cards and/or keys should be limited.
· Limit time that controlled substances are out.
· Never leave controlled substances unattended.
· Only allow authorized users to handle them.
· Never take controlled substances out of the secured area.
Pharmaceuticals taken into the field must also be stored under appropriate conditions with restricted or limited access. Contact the IACUC (iacuc@uwyo.edu) or AV (lkendal2@uwyo.edu) for advice on obtaining a suitable portable storage container and documentation for transport.
4. Disposal of Controlled Substances
All expired or unused materials must be securely locked and segregated as described in Section 3. The PI is responsible for disposing of these materials per federal, state, and UW regulations and cannot transfer them to another PI. If the lab is closing or the PI is retiring, they must dispose of all controlled substances before closing the lab or letting the DEA registration expire. Contact the Safety Office at uwehs@uwyo.edu for further information on disposal.
5. Reporting Theft or Loss of Controlled Substances
If you suspect a theft, immediately notify the UW Police Department (307-766-5179). In addition, federal law requires you report any incidents the Drug Enforcement Administration (DEA) within 24 hours of a loss (use DEA Form 106).
6. Recordkeeping of Controlled Substances
Logbooks and records should be separated from other records and kept near the controlled substance work area. These records must be legible. Events of loss, destruction, or theft must be recorded in detail as well (and reported as stated above). All records and logbooks must be retained for at least two years from the last entry.
6.1. Receipt of Controlled Substances
These records should indicate date received, name of supplier, substance name and description, amount received, and authorized person handling the substance. Copies of order forms must be stored in a securely locked cabinet. Controlled Substances Receipt Form
6.2. Use Log of Controlled Substance
Each controlled substance should have its own use log. Different substances shouldn’t be recorded interchangeably. Controlled Substances Use Form
6.3. Inventory of Controlled Substance
The complete and accurate inventory of controlled substances stocked in each registrant’s lab should be conducted initially when registered and biennially (every two years). These records must be consistent with records of receipt, use, and disposal. Controlled Substances Inventory Form.
Note: The information provided above is not intended to cover all of the DEA regulations governing Controlled Substances. Further information on requirements for managing and handling controlled substances can be found on the DEA website. For more information contact iacuc@uwyo.edu or uwehs@uwyo.edu .
6.4. FORMs – recordkeeping
· Controlled Substances Receipt Form
Please use the link below and fill out the form to the best of your ability. Per UW policies, you are protected from retaliation for voicing your concerns, but please be aware that there is always some risk for reporting. The link will allow you to remain anonymous if you choose to do so. but anonymous complaints may make it more difficult to provide additional information to better help investigate these claims.